APIIDAE
M. J. Donoghue et P. D. Cantino
Donoghue et Cantino in Taxon 56: E31. Aug 2007
[Campanulales+[Escalloniaceae+[Bruniales+Dipsapiidae]]]
CAMPANULALES Juss. ex Bercht. et
J. Presl
Berchtold et Presl, Přir. Rostlin: 253. Jan-Apr
1820 [‘Campanulaceae’]
Habit Usually bisexual
(sometimes monoecious, gynomonoecious, andromonoecious, polygamomonoecious,
dioecious, gynodioecious, or androdioecious), usually perennial, biennial or
annual herbs (sometimes evergreen, rarely deciduous, suffrutices, shrubs, trees
or lianas). A large number of species are xerophytes and some species are
succulent; others are aquatic or helophytes. C4 and/or CAM
physiology sometimes present.
Vegetative anatomy Phellogen
ab initio usually superficial (sometimes cortical). Vascular tissue
atactostele-like, with scattered stem bundles. Cortical and/or medullary
vascular bundles sometimes present. Endodermis sometimes with thick-walled
cells. Cambium storied or non-storied. Secondary lateral growth usually normal
or absent (sometimes anomalous via concentric cambia or cylindrical cambium).
Vessel elements usually with simple (sometimes scalariform or reticulate)
perforation plates; lateral pits usually alternate (sometimes opposite, rarely
scalariform), usually bordered (sometimes simple) pits. Vestured pits present.
Imperforate tracheary xylem elements libriform fibres, tracheids or fibre
tracheids with simple or bordered pits, septate or non-septate (often also
vasicentric tracheids). Wood rays uniseriate or multiseriate, homocellular or
heterocellular, or absent. Axial parenchyma apotracheal diffuse or absent, or
paratracheal scanty, aliform, lozenge-aliform, winged-aliform, confluent,
vasicentric, or banded (sometimes absent). Wood elements sometimes storied.
Tyloses sometimes frequent. Intraxylary phloem rarely? present. Sieve tube
plastids S type. Nodes usually ≥3:≥3, trilacunar, pentalacunar or
multilacunar with three or more leaf traces (rarely 1:1, unilacunar with one
trace). Phloem sometimes with articulated laticifers containing coloured, white
or colourless latex rich in triterpenes and/or tissues with schizogenous
secretory resinous canals often lined with epithelial cells (scattered latex
cells sometimes present as well as resinous canals). Sclerenchymatous
idioblasts often present. Parenchyma sometimes with prismatic calciumoxalate
crystals (acicular crystals, druses, styloids, crystal sand, etc.).
Trichomes Hairs unicellular or
multicellular, uniseriate or biseriate, simple or branched, flagellar hairs,
T-shaped, stellate, candelabra-shaped, dendritic, arachnoid, peltate, lepidote,
or vesicular; glandular hairs with unicellular or multicellular stalk and
unicellular or multicellular head present or absent; laticiferous hairs
sometimes present.
Leaves Usually alternate
(spiral or rarely distichous; sometimes opposite, rarely verticillate), usually
simple (sometimes pinnately or palmately compound), entire or pinnately lobed
(sometimes repeatedly pinnately lobed), sometimes coriaceous, with
conduplicate, supervolute, revolute or involute ptyxis. Stipules absent; leaf
sheath usually absent. Petiole vascular bundle transection usually arcuate
(sometimes annular). Venation pinnate, palmatopinnate, parallelopinnate,
parallelodromous, craspedodromous, semicraspedodromous, camptodromous or
palmate (rarely flabellate or acrodromous). Stomata usually anomocytic
(sometimes anisocytic or helicocytic, rarely paracytic). Cuticular wax
crystalloids when known partly as large glabrous more or less inrolled scales,
partly as reticulate to annular threads or small scales (sometimes rodlets or
platelets). Secretory cavities (with resin and/or latex) present or absent.
Mesophyll sometimes with idioblasts containing sclereids. Leaf margin serrate,
serrate-dentate, crenate, lobate or entire, sometimes glandular serrate or with
hydathodes. Extrafloral nectaries rarely present.
Inflorescence Terminal or
axillary, fasciculate, paniculate, corymbose, raceme-, spike-, head- or
umbel-like, or raceme, dense capitula, or capitulate pseudanthia with involucre
of bracts (rarely helicoid cyme; flowers sometimes solitary axillary, rarely
solitary terminal).
Flowers Actinomorphic or
zygomorphic (sometimes resupinate). Hypanthium sometimes present. Usually
epigyny (rarely half epigyny or hypogyny). Sepals (two to) five (to eight),
with valvate, imbricate or open aestivation, usually persistent, often connate,
or modified into usually accrescent scales or hairs, pappus, or absent. Petals
(three to) five (to ten), usually with valvate (rarely imbricate or open)
aestivation, usually caducous, usually more or less connate into tubular,
urceolate, campanulate, infundibuliform, spathulate, or (uni- or) bilabiate
(rarely free) corolla, sometimes fringed (rarely absent), with apex inflexed.
Nectariferous or non-nectariferous disc intrastaminal, annular, scale-like,
tubular, or absent (sometimes with nectariferous glands alternating with
stamens).
Androecium Stamens (two to)
five (to ten), haplostemonous, antesepalous, alternipetalous. Filaments usually
free from each other (sometimes connate), usually adnate to petals
(epipetalous). Anthers free (sometimes connivent) or connate into tube around
style, basifixed or dorsifixed, usually non-versatile, usually tetrasporangiate
(rarely disporangiate), usually introrse, longicidal (dehiscing by longitudinal
slits); introrse anthers in Asteraceae and in some Goodeniaceae and Campanulaceae entirely or
partially connate, syngenesy. Tapetum ab initio cellular, with multinucleate
cells, later usually amoeboid-periplasmodial by dissolution of cell walls, or
secretory with binucleate or multinucleate cells. Female flowers with
staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3(–6)-colporate
(sometimes tri- to polycolpate, rarely tri- to polyporate), usually shed as
monads (rarely tetrads), usually tricellular (sometimes bicellular) at
dispersal. Exine tectate or semitectate, with columellate infratectum,
punctate, perforate, microreticulate, or lophate, often caveate (fenestrate;
sometimes reticulate, rugulate or striate, rarely imperforate), echinate,
verrucate, spinulate, scabrate, psilate, or granulate.
Gynoecium Pistil composed of
(one or) two (to five) connate carpels. Ovary usually inferior (rarely
semi-inferior or superior), unilocular or bilocular (to quinquelocular),
sometimes incompletely septate. Style single, usually bilobate or simple
(rarely trilobate), usually hairy. Stigma(s) capitate, truncate, clavate,
discoid, or stigma bilobate (rarely trilobate or quinquelobate), often with
stigmatic surface on adaxial side of lobes (sometimes with appendages on apex
of stylar branches), usually papillate, Dry or Wet type. Male flowers with
pistillodium. Secondary pollen display with several different types of stylar
adaptations present in Asteraceae, Calyceraceae, Goodeniaceae and Campanulaceae, such as pollen
pump mechanisms, plunger or brush pollination, pollen collecting hairs, cupular
structure outside gradually and strongly expanding style, and stigma late
pollen receptive.
Ovules Placentation basal or
axile (sometimes apical, rarely parietal or free central). Ovule one per ovary,
or two to numerous per carpel, usually anatropous (rarely campylotropous or
hemianatropous), ascending (sometimes pendulous or horizontal), epitropous
(sometimes apotropous), unitegmic, tenuinucellar. Integument rarely
vascularized. Hypostase absent. Endothelium present. Archespore usually
unicellular (sometimes multicellular, rarely bicellular). Megagametophyte
usually monosporous, Polygonum type (rarely disporous or
tetrasporous). Synergids sometimes with a filiform apparatus. Antipodal cells
usually ephemeral (sometimes persistent, occasionally proliferating and
haustorial). Endosperm development usually cellular (rarely nuclear). Endosperm
haustoria chalazal and/or micropylar, or absent. Embryogenesis usually asterad
(sometimes chenopodiad or solanad). Agamospermy sometimes abundant.
Fruit A loculicidal (sometimes
septicidal, poricidal or irregularly dehiscing) capsule, berry, drupe or achene
(cypsela) with seed wall adnate to pericarp and with persistent and accrescent
calyx (rarely a schizocarp with nutlike mericarps or a pyxidium).
Seeds Aril absent. Exotestal
cells often thickened, usually palisade, sometimes flattened, cuboidal,
fibriform or indistinct. Endotesta often developed into endothelium,
integumentary tapetum. Perisperm not developed. Endosperm copious to sparse,
oily and proteinaceous (starch usually absent; sometimes with hemicellulose or
absent). Embryo large or small, usually well developed, straight, without
chlorophyll. Embryo suspensor often filamentous. Cotyledons usually two (rarely
absent). Germination phanerocotylar.
Cytology n = 6–18 (rarely 2,
or up to 120; 9 and 17 most frequent) – Polyploidy, aneuploidy and
agamospermy frequently occurring. Protein bodies present in nucleus.
DNA Plastid genome with large
number of inversions. Mitochondrial gene rpl2 lost.
Phytochemistry Flavonols
(kaempferol, quercetin), O-methylflavonols, afzelechin, flavonoid
sulfates, biflavonoids, cyanidin, coumarins, p-coumaric acid,
dammaranes, Group VI secoiridoids (e.g. secologanin), Group VII secoiridoids
(e.g. sweroside), Group X secoiridoids (e.g. loganin), cantleyoside, oleanolic
acid derivatives, diterpenoids, lupeol and its derivative lupenylacetate,
pentacyclic triterpene alcohols, terpenoid ethereal oils, balsams, triterpene
acetates, furanoeremophilane sesquiterpenes, sesquiterpene lactones, ursolic
acid, caffeic acid esters (verbascosides), chlorogenic acid, ellagic acid
(rare), tannins, proanthocyanidins (prodelphinidins, rare), pyrrolizidine
alkaloids as macrocyclic aliphatic monocarboxylic diesters, iridoid alkaloids,
caurane alkaloids and other alkaloids (rarely benzylisoquinoline alkaloids,
homoerythrine alkaloids: homoerythrine, homoerythroidine, homoazaerythrine,
holidine, etc.), cyanogenic glycosides (linamarin, lotaustralin,
proacacipetalin, prunasin, sambunigrin, triglochinin, zierin), phenylalanine-
and tyrosine-derived cyanogenic compounds (rare), triterpene saponins, fatty
acid derived polyacetylenes (substitute for iridoids?), aliphatic
tetrahydropyrane derivatives, amides, asarone, germacrane-like compounds,
myo-inisitol, chiro-inositols (pinitol, quebrachitol),
lignans (pinoresinol), arbutin, sinapic acid, chelidonic acid,
polyacetate-derived arthroquinones, stigmasterol, polysterols, steroids, and
stearic acid. Carbohydrates usually stored as oligo- or polyfructosans (i.a.
inulin in subterranean parts of perennials) with isokestose linkages (starch
usually absent).
Systematics Campanulales are sister to the
clade [Escalloniaceae+[Bruniales+Dipsapiidae]]
(Tank & Donoghue 2010).
One possible topology of Campanulales is as follows:
[[Rousseaceae+Campanulaceae]+ [Pentaphragmataceae+[[Stylidiaceae+[Phellinaceae+[Argophyllaceae+Alseuosmiaceae]]]+ [Menyanthaceae+[Goodeniaceae+[Calyceraceae+Asteraceae]]]]]].
Rousseaceae and Campanulaceae share the
characters large flowers and free stamens (Stevens 2001 and onwards). Campanulales except Rousseaceae and Campanulaceae have the
potential synapomorphy corolla lobes provided with marginal wings.
The clade [Phellinaceae+[Alseuosmiaceae+Argophyllaceae]] is
characterized by the synapomorphies: woodiness; lamina serrate and
gland-toothed; and x = 8. Moreover, Phelline and Argophyllaceae share,
according to Stevens (2001 onwards): subepidermal phellogen; spiny pollen
grains, with rugulate exine; short style; and apotropous ovules.
The clade [Menyanthaceae+[Goodeniaceae+[Calyceraceae+Asteraceae]]] have the following
potential synapomorphies: vessel elements with simple perforation plates;
inflorescence with one terminal flower, single flowers and cymes below; petals
connate, with early tube formation and with strong fused marginal (commissural)
veins joining median vein near apex; filaments epipetalous; tapetum with bi- or
multinucleate cells; pollen grains psilate; pistil composed of two carpels;
integument more than ten cell layers thick; antiraphal vascular bundle
proceeding to micropyle; absence of endosperm haustoria; embryo long; x = 9;
and presence of inulin and caffeic acid.
The clade [Goodeniaceae+[Calyceraceae+Asteraceae]] share the unique
features: pollen grains with bifurcating columellae; stigma papillate, Dry
type; secondary pollen presentation (protandry, anthers connivent at
dehiscence, style elongating following pollen deposition); calyx persistent in
fruit; and x = 8. Finally, Calyceraceae and Asteraceae possess the
synapomorphies (Stevens 2001 onwards): involucrate and capitate inflorescence
(forming pseudanthium); small sessile flowers; tubular corolla, with connate
commissural veins (median veins sometimes absent); presence of filament collar;
presence of pollenkitt; pollen grains with intercolpal depressions; pistil
composed of a single carpel; unilocular ovary; fruit a cypsela, with persistent
and modified calyx, involved in fruit dispersal.
Airy Shaw in Kew Bull. 18: 249. 8 Dec 1965
Alseuosmiales Doweld,
Tent. Syst. Plant. Vasc.: liv. 23 Dec 2001;
Platyspermatiaceae Doweld, Tent. Syst. Plant. Vasc.:
liv. 23 Dec 2001 [’Platyspermataceae’];
Alseuosmiineae Reveal in Kew Bull. 66: 47. Mar
2011
Genera/species 5/10
Distribution New Guinea,
southeastern Australia, Queensland, New Caledonia, New Zealand.
Fossils Unknown.
Habit Usually bisexual (in
Wittsteinia sometimes functionally unisexual), evergreen shrubs
(sometimes epiphytic).
Vegetative anatomy Phellogen
ab initio superificial. Endodermis present, uniseriate. Young stem with
separate vascular bundles. Vessel elements usually with scalariform (sometimes
also simple) perforation plates; lateral pits usually opposite or alternate
(sometimes scalariform, with numerous cross-bars), bordered pits. Imperforate
tracheary xylem elements tracheids with simple or bordered pits, septate or
non-septate (as mature living and starchy). Wood rays usually absent (in
Crispiloba uniseriate to multiseriate, heterocellular; in
Wittsteinia sometimes multiseriate). Axial parenchyma usually absent
(sometimes apotracheal or paratracheal scanty). Starch-storing fibres usually
present. Pericyclic fibres weakly developed. Sieve tube plastids S type? Nodes
3:3, trilacunar with three leaf traces. Calciumoxalate crystals not found.
Trichomes Hairs unicellular,
bicellular or multicellular, uniseriate (in Platyspermation with
reddish persistent base), usually confined to leaf axils (in
Platyspermation also on other parts of plant).
Leaves Alternate (spiral),
simple, entire, sometimes coriaceous, with conduplicate ptyxis. Stipules and
leaf sheath absent. Tufts of uniseriate multicellular hairs with dark red
pigment present in leaf axils. Petiole vascular bundle transection arcuate;
petiole usually with uniseriate endodermis. Venation pinnate. Stomata
anomocytic. Cuticular wax crystalloids? Sclereids usually present. Leaf margin
serrate or entire.
Inflorescence Usually axillary
(sometimes terminal), fasciculate, raceme- or umbel-like, cymose, or flowers
solitary axillary.
Flowers Actinomorphic.
Hypanthium sometimes present. Usually epigyny (sometimes half epigyny). Sepals
(four or) five (to seven), with valvate or open aestivation, free. Petals (four
or) five (to seven), with valvate aestivation, connate into urceolate,
campanulate or infundibuliform corolla; corolla lobe margins erose, with
fimbriate or notched wings (absent in Platyspermation). Nectariferous
disc intrastaminal or absent. Tanniniferous cells present in flowers.
Androecium Stamens (four or)
five (to seven), haplostemonous, antesepalous, alternipetalous. Filaments free
from each other, usually adnate to corolla tube (epipetalous, sometimes only at
base). Anthers basifixed to almost dorsifixed, non-versatile, tetrasporangiate,
usually introrse, longicidal (dehiscing by longitudinal slits). Tapetum
secretory, with binucleate cells. Staminodia present in functional female
flowers in Wittsteinia. Secondary pollen display absent.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolpate or tricolporate,
usually shed as monads (sometimes tetrads), tricellular at dispersal. Exine
tectate, with columellate intratectum, microreticulate to punctate?,
tuberculate.
Gynoecium Pistil composed of
two (or three) carpels. Ovary usually inferior (sometimes semi-inferior),
usually bilocular (sometimes trilocular). Style single, simple, narrow or
stout. Stigma capitate, clavate or discoid, often bilobate (sometimes
trilobate), little expanded, type? Pistillodium present in functional male
flowers in Wittsteinia.
Ovules Placentation axile.
Ovules two to numerous (sometimes one) per carpel, anatropous, unitegmic,
tenuinucellar. Integument ? cell layers thick. Megagametophyte monosporous,
Polygonum type. Endosperm development cellular. Endosperm haustoria?
Embryogenesis?
Fruit Usually a berry (in
Platyspermation a capsule), usually with persistent calyx.
Seeds Seeds small (in
Platyspermation flattened). Aril absent. Exotestal cells little
thickened, lignified (Alseuosmia). Mesotesta persistent. Endotesta?
Perisperm not developed. Endosperm copious. Embryo small, straight,
chlorophyll? Cotyledons two. Germination?
Cytology n = 9
(Alseuosmia)
DNA Mitochondrial gene
rpl2 absent?
Phytochemistry Insufficiently
known. Flavonols (quercetin, kaempferol), p-coumaric acid, lupeol and
its derivative lupenylacetate, triterpene acetates, condensed tannins,
ellagitannins, proanthocyanidins, caffeic acid, triterpene saponins,
stigmasterol, and stearic acid present (Alseuosmia). Inulin? Iridoids
and alkaloids not found.
Use Ornamental plants.
Systematics Platyspermation
(1; P. crassifolium; New Caledonia); Crispiloba (1; C.
disperma; northeastern Queensland), Alseuosmia
(5; A. banksii, A. macrophylla, A. pusilla, A.
quercifolia, A. turneri; New Zealand), Wittsteinia (2;
W. papuana: Papua New Guinea; W. vacciniacea: eastern
Victoria; incl. Periomphale?),
Periomphale
(1; P. balansae; New Caledonia; in Wittsteinia?).
Alseuosmiaceae are
sister-group to [Argophyllaceae].
Platyspermation
is sister to the remaining Alseuosmiaceae. The corolla
tube is very short in Platyspermation,
and the lobes are reflexed and provided with papillae along the margins. Tufts
of uniseriate hairs are particularly abundant in the leaf axils, as in other
Alseuosmiaceae, but
in Platyspermation
uniseriate hairs cover practically the entire plant. The reddish hair bases
somewhat resemble glands.
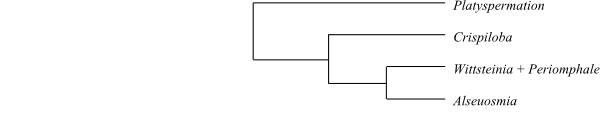 |
Cladogram of Alseuosmiaceae based
on morphology and DNA sequence data (Kårehed & al. 1999).
|
Takhtajan, Sist. Magnoliof. [Systema
Magnoliophytorum]: 208. 24 Jun 1987
Corokiaceae Kapil ex
Takht., Divers. Classif. Fl. Pl.: 374. 24 Apr 1997
Genera/species 2/22
Distribution Eastern
Australia, New Caledonia, New Zealand, Lord Howe, Rapa Island.
Fossils Unknown.
Habit Bisexual, evergreen
trees or shrubs. Often densely tomentose.
Vegetative anatomy Phellogen
ab initio subepidermal? (Corokia). Vessel elements with scalariform
perforation plates (often with numerous cross-bars); lateral pits usually
alternate (sometimes opposite), bordered pits. Imperforate tracheary xylem
elements tracheids (absent in Argophyllum and some species of
Corokia) with simple and/or bordered pits, septate or non-septate
(also vasicentric tracheids). Wood rays uniseriate or multiseriate,
heterocellular. Axial parenchyma usually absent (rarely apotracheal diffuse, or
paratracheal scanty). Sieve tube plastids S type? Nodes usually 3:3, trilacunar
with three leaf traces (in Corokia sometimes 1:1, unilacunar with one
trace; in Argophyllum sometimes 5:5, pentalacunar with five traces).
Crystals absent.
Trichomes Hairs T-shaped,
usually with multicellular uniseriate stalk (provided with slits) and very long
terminal bifid cell with narrowing apices.
Leaves Alternate (spiral),
simple, entire, with supervolute-curved ptyxis (Corokia macrocarpa).
Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate.
Venation pinnate. Stomata anomocytic, with guard cells raised above epidermis.
Cuticular wax crystalloids? Leaf margin coarsely glandular serrate to entire
(in Corokia entire).
Inflorescence Terminal or
axillary, panicle or corymb (Argophyllum) or paniculate, raceme-shaped
or few-flowered fasciculate (Corokia), or flowers solitary axillary
(Corokia).
Flowers Actinomorphic. Half
epigyny (Argophyllum) or epigyny (Corokia). Sepals (four or)
five (to eight), with valvate aestivation, persistent, connate at base. Petals
(four or) five (to eight), with valvate aestivation, sometimes with wings at
margins, free or connate at base; each corolla lobe usually with adaxial
fringed ligule immediately above corolla tube mouth (absent in Corokia
macrocarpa). Nectariferous disc present in Corokia. Tanniniferous
cells present in flowers.
Androecium Stamens (four or)
five (to eight), haplostemonous, antesepalous, alternipetalous. Filaments free
from each other and from tepals. Anthers dorsifixed, versatile?,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum secretory. Staminodia absent.
Pollen grains Micosporogenesis
simultaneous? Pollen grains tricolporate, shed as monads, bicellular
(Argophyllum) or tricellular (Corokia) at dispersal.
Endoapertures complex and H-shaped. Exine tectate, with columellate?
infratectum, finely perforate, coarsely scabrate (Argophyllum) or
spinulate (Corokia).
Gynoecium Pistil composed of
(one or) two or three (to five) connate carpels. Ovary semi-inferior or
inferior, bilocular or trilocular (to quinquelocular) (Argophyllum),
or unilocular or bilocular (to quinquelocular) (Corokia). Style
single, simple, short. Stigma capitate, punctate, or bilobate or trilobate (to
quinquelobate), papillate?, Wet type. Pistillodium absent.
Ovules Placentation axile
(Argophyllum) or apical-axile (Corokia). Ovule one
(Corokia) or several (Argophyllum) per carpel, anatropous,
pendulous (Corokia), apotropous, unitegmic, tenuinucellar. Integument
approx. six cell layers thick. Megasporangium with massive base.
Megagametophyte monosporous, Polygonum type. Endosperm development
cellular (Corokia). Endosperm haustoria chalazal and micropylar
(Corokia). Embryogenesis chenopodiad (Corokia).
Fruit A loculicidal capsule
(Argophyllum) or a unilocular or bilocular drupe with persistent calyx
and style (Corokia).
Seeds Aril? Exotestal cells
with inner walls heavily thickened and lignified (Argophyllum), or all
walls somewhat thickened (Corokia). Endotesta? Perisperm not
developed. Endosperm fleshy, with hemicellulose. Embryo small
(Argophyllum) or medium-sized (Corokia), straight,
chlorophyll? Cotyledons two. Germination?
Cytologi n = 9
(Corokia)
DNA Mitochondrial gene
rpl2 absent?
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, Group VI secoiridoids (secologanin),
triterpenes, ellagic and gallic acid, tannins, and caffeic acid, present.
Inulin? Nickel accumulated in two species of Argophyllum.
Use Ornamental plants.
Berchtold et Presl, Přir. Rostlin: 254. Jan-Apr
1820 [‘Astereae’], nom. cons.
Cichoriaceae Juss., Gen.
Plant.: 168. 4 Aug 1789 [‘Cichoraceae’], nom. cons.;
Compositae Giseke, Prael. Ord. Nat. Plant.: 538. Apr
1792, nom. cons. et nom. alt.; Cynarineae Raf., Anal.
Nat.: 191. Apr-Jul 1815 [‘Cynarea’];
Cnicaceae Vest, Anleit. Stud. Bot.: 273, 297. 1818
[’Cnicoideae’]; Tanacetaceae Vest,
Anleit. Stud. Bot.: 273, 298. 1818 [’Tanacetoideae’];
Xanthiaceae Vest, Anleit. Stud. Bot.: 273, 298. 1818
[‘Xanthoideae’]; Adenostylidaceae
Bercht. et J. Presl, Přir. Rostlin: 254. Jan-Apr 1820
[‘Adenostyleae’], nom. illeg.;
Ambrosiaceae Bercht. et J. Presl, Přir. Rostlin:
254. Jan-Apr 1820, nom. cons.; Anthemidaceae Bercht.
et J. Presl, Přir. Rostlin: 254. Jan-Apr 1820 [‘Anthemideae’];
Arctotidaceae Bercht. et J. Presl, Přir. Rostlin:
255. Jan-Apr 1820 [‘Arctotideae’];
Artemisiaceae Martinov, Tekhno-Bot. Slovar: 48. 3 Aug
1820 [’Artemisiae’]; Athanasiaceae
Martinov, Tekhno-Bot. Slovar: 56. 3 Aug 1820 [’Athanasiae’];
Calendulaceae Bercht. et J. Presl, Přir. Rostlin:
254. Jan-Apr 1820; Carduaceae Bercht. et J. Presl,
Přir. Rostlin: 255. Jan-Apr 1820; Centaureaceae
Bercht. et J. Presl, Přir. Rostlin: 255. Jan-Apr 1820
[‘Centaureae’]; Echinopaceae Bercht. et
J. Presl, Přir. Rostlin: 255. Jan-Apr 1820 [‘Echinopseae’];
Eupatoriaceae Bercht. et J. Presl, Přir. Rostlin:
253. Jan-Apr 1820 [‘Eupatoriae’];
Helianthaceae Bercht. et J. Presl, Přir. Rostlin:
254. Jan-Apr 1820; Inulaceae Bercht. et J. Presl,
Přir. Rostlin: 254. Jan-Apr 1820; Lampsanaceae
Martinov, Tekhno-Bot. Slovar: 356. 3 Aug 1820 [‘Lampsana’], nom.
illeg.; Picridaceae Martinov, Tekhno-Bot. Slovar:
482. 3 Aug 1820 [‘Picrides’];
Santolinaceae Martinov, Tekhno-Bot. Slovar: 560. 3
Aug 1820 [‘Santolinae’]; Senecionaceae
Bercht. et J. Presl, Přir. Rostlin: 254. Jan-Apr 1820
[‘Senecioneae’]; Serratulaceae Martinov,
Tekhno-Bot. Slovar: 577. 3 Aug 1820 [’Serratulae’];
Tussilag[in]aceae Bercht. et J. Presl, Přir.
Rostlin: 254. Jan-Apr 1820 [‘Tussilagineae’];
Heleniaceae Raf. in Cincinnati Lit. Gaz. 2: 28. 24
Jul 1824 [‘Helenidia’]; Acarnaceae Link,
Handbuch 1: 684. 20 Apr 1829, nom. illeg.;
Ambrosiales Link, Handbuch 1: 816. 4-11 Jul 1829
[‘Ambrosiaceae’]; Anthemidales Link,
Handbuch 1: 752. 4-11 Jul 1829 [’Anthemideae’];
Asterales Link, Handbuch 1: 731. 4-11 Jul 1829
[’Asteroideae’]; Calendulales Link,
Handbuch 1: 776. 4-11 Jul 1829 [’Calendulaceae’];
Cichoriales Link, Handbuch 1: 779. 4-11 Jul 1829
[’Cichoriaceae’]; Coreopsidaceae Link,
Landbuch 1: 786, 20 apr 1829 [‘Coreopsideae’], nom. illeg.;
Echinopales Link, Handbuch 1: 814. 4-11 Jul 1829
[‘Echinopeae’]; Helichrysaceae Link,
Handbuch 1: 712. 20 Apr 1829 [‘Elichryseae’], nom. illeg.;
Partheniaceae Link, Handbuch 1: 816. 20 Apr 1829,
nom. illeg.; Perdiciaceae Link, Handbuch 1: 728. 20
Apr 1829 [‘Perdicieae’], nom. illeg.;
Eupatoriineae Link, Handbuch 1: 729. 4-11 Jul 1829;
Gnaphaliaceae Link ex F. Rudolphi, Syst. Orb. Veg.:
46. 5-12 Jul 1830 [‘Gnaphalieae’];
Cynaraceae Spenn., Handb. Angew. Bot. 1: 296. 1-19
Jul 1834 [‘Cynareae’]; Mutisiaceae
Burnett, Outl. Bot.: 934, 935, 1094, 1111. Feb 1835;
Asterineae Burnett, Outlines Bot.: 901, 1111. Feb
1835 [‘Asterosae’]; Nassauviaceae
Burmeist., Handb. Naturgesch. 1: 290. 12-17 Dec 1836;
Vernoniaceae Burmeist., Handb. Naturgesch. 1: 296.
12-17 Dec 1836; Aposeridaceae Raf., New Fl. N. Amer.
4: 106. med 1838 [’Aposerides’];
Asteropsida Brongn., Enum. Plant. Mus. Paris: xvii,
32. 12 Aug 1843 [’Asteroideae’];
Xeranthemaceae Döll, Rhein. Fl.: 498. 24-27 Mai 1843
[‘Xeranthemeae’]; Matricariaceae Voigt,
Hort. Suburb. Calcutt.: 400. Aug-Dec 1845;
Cichoriineae J. Presl in Nowočeská Bibl.
[Wšobecný Rostl.] 7: 856, 944. 1846 [‘Cichoraceae’];
Mutisiineae J. Presl in Nowočeská Bibl. [Wšobecný
Rostl.] 7: 855, 941. 1846; Nassauviineae J. Presl in
Nowočeská Bibl. [Wšobecný Rostl.] 7: 856, 943. 1846;
Senecionineae J. Presl in Nowočeská Bibl.
[Wšobecný Rostl.] 7: 855, 877. 1846 [‘Senecionideae’];
Vernoniineae J. Presl in Nowočeská Bibl.
[Wšobecný Rostl.] 7: 855, 856. 1846 [‘Vernonieae’];
Lactucaceae Drude in J. A. Schenk, Handb. Bot. 3(2):
369. 1886; Carduales Small, Fl. S.E. U.S.: 1148. 22
Jul 1903; Grindeliaceae Reichb. ex A. Heller in
Muhlenbergia 2: 330. 30 Dec 1907; Madiaceae (Greene)
A. Heller in Muhlenbergia 2: 332. 30 Dec 1907
Genera/species
1.637/32.800–>33.200?
Distribution Cosmopolitan
except Antarctica.
Fossils A fossil
infructescence, Raiguenrayun cura, was reported from Eocene layers in
Patagonia in Argentina (Barreda & al. 2010, 2012; Panero & al. 2014).
Pollen fossils are known from the Paleocene to the Eocene of South Africa
(Tubulifloridites antipodica), the Eocene of British Columbia and from
the Oligocene onwards of North and South America, India, Africa, Australia,
Tasmania (Mutisiapollis patersonii), East Asia, and Europe, although
pollen possibly originating from Asteraceae have been found in
Paleocene layers of western South America.
Habit Usually bisexual
(sometimes monoecious, gynomonoecious, polygamomonoecious, dioecious,
androdioecious, or gynodioecious), usually perennial, biennial or annual herbs
(sometimes evergreen, rarely deciduous, shrubs, trees or lianas). Some species
are succulent. Numerous representatives are spiny or prickly. Many Asteraceae have stem or root
nodules or lignotuber. A large number of species are xeromorphous. Some species
have C4 and/or CAM physiology. Often with a strong scent or
odour.
Vegetative anatomy Phellogen
ab initio usually superficial (sometimes deeply seated). Cortical and/or
medullary vascular bundles sometimes present. Medulla in some species of
Senecioneae stratified by diaphragms. Vascular bundles usually
separated (in woody species cylinder). Cambium often storied. Wood elements
sometimes storied. Secondary lateral growth usually normal (also in numerous
herbaceous representatives; sometimes anomalous from concentric cambia or a
cylindrical cambium). Vessel elements usually with simple (sometimes
scalariform or reticulate) perforation plates; lateral pits usually alternate
(sometimes opposite), usually bordered (sometimes simple) pits. Vestured pits
present. Imperforate tracheary xylem elements libriform fibres with small
simple pits, septate or non-septate (often also vasicentric tracheids). Wood
rays uniseriate or multiseriate, homocellular or heterocellular. Axial
parenchyma apotracheal diffuse, or paratracheal scanty, aliform,
lozenge-aliform, winged-aliform, confluent, vasicentric, or banded. Tyloses
sometimes frequent. Intraxylary phloem rarely? present. Sieve tube plastids S
type. Endodermis sometimes with thick-walled cells. Nodes >3:>3, trilacunar, pentalacunar
or multilacunar with three or more? leaf traces (rarely unilacunar with one?
trace). Phloem (especially in Lactuceae) with articulated laticifers
containing latex rich in triterpenes, and/or tissues with schizogenous
secretory resinous ducts often lined with epithelial cells (scattered latex
cells sometimes present as well as resinous ducts). Parenchyma in some species
with prismatic calciumoxalate crystals; acicular crystals, styloids, crystal
sand and other types of crystals sometimes present.
Trichomes Hairs unicellular or
multicellular, uniseriate or biseriate, simple or branched, flagellar hairs,
T-shaped, stellate, candelabra-shaped, dendritic, arachnoid, peltate, lepidote,
or vesicular; glandular hairs with unicellular or multicellular head; sometimes
laticiferous hairs.
Leaves Usually alternate
(spiral; sometimes opposite, rarely verticillate), usually simple (sometimes
pinnately or palmately? compound), entire or pinnately lobed (sometimes
repeatedly pinnately lobed), often with conduplicate or revolute ptyxis.
Stipules and leaf sheath absent. Petiole vascular bundle transection usually
arcuate. Venation pinnate, palmatopinnate, parallelopinnate, parallelodromous,
or palmate. Stomata usually anomocytic (sometimes anisocytic or helicocytic).
Cuticular wax crystalloids partly as large glabrous partially inrolled scales,
partly as reticulate to annular threads or small scales, or as rosettes of
platelets (Fabales type). Secretory cavities and ducts (with resin and
latex) present or absent. Leaf margin serrate, often serrate-dentate, crenate,
lobate or entire, sometimes with hydathodes. Extrafloral nectaries rarely
present.
Inflorescence Terminal or
axillary, primarily racemose capitulum (pseudanthium) with abaxial involucre
consisting of one or several rows of bracts. Capitulum with one or more sessile
flowers on common flat to conical or cylindrical receptacle (sometimes, i.a. in
Heliantheae, with one bract [floral bract] present adjacent to each
flower or with prickles or bristles, e.g. in Carduoideae). Capitula
usually organized in compound, often corymbose inflorescence or sometimes in
cymose secondary compound capitula (sometimes with secondary involucre;
capitulum sometimes consisting of one flower, many capitula aggregated into
“super-capitulum”). Capitula homogamous, with all flowers usually bisexual,
or heterogamous, with external flowers, ray florets, female, sterile or
bisexual, and central flowers, disc florets, bisexual or functionally male.
Capitulum without terminal flower, with usually centripetal (peripheral flowers
in, e.g., Gorteria centrifugal) development of flowers. Floral
prophylls (bracteoles) absent. Extrafloral nectaries sometimes present on
bracts.
Flowers Actinomorphic or
zygomorphic, usually small. Epigyny. Sepals (and petal bases) modified into
persistent and usually accrescent pappus (hairs, scales or bristles), or
absent. Petals (three to) five (or six), with valvate aestivation, tubular,
spatulate (apically tridentate or quinquedentate) or bilabiate, with upper lip
unilobate and lower lip quadrilobate, or upper lip bilobate and lower lip
trilobate, connate (rarely absent), usually without midvein (present in, e.g.,
Barnadesioideae); corolla tube development early or late.
Nectariferous disc intrastaminal, annular, scale-like or tubular, surrounding
stylar base.
Androecium Stamens (three to)
five (or six), haplostemonous, antesepalous, alternipetalous. Filaments usually
free from each other (rarely connate into tube), adnate to corolla tube
(epipetalous), in upper part usually with ring of thick-walled cells. Anthers
usually caudate, with conspicuous apical and basal (calcarate) appendages,
usually connate into tube around style, basifixed or dorsifixed, non-versatile,
usually tetrasporangiate (rarely disporangiate), introrse, longicidal
(dehiscing by longitudinal slits). Tapetum ab initio cellular, with
multinucleate cells, later usually amoeboid-periplasmodial by dissolution of
cell walls (rarely secretory). Female flowers usually with staminodia.
Secondary pollen display present (many species with “pollen pump” or
“pollen brush”).
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (sometimes
triporate, rarely pantoporate; in Cichorieae usually strongly lophate,
fenestrate; pollen grains often caveate in lineages basal to Corymbium
and Asteroideae, but also in Senecioneae,
Arctotideae and some Cichorieae), shed as monads, tricellular
at dispersal. Exine tectate or semitectate, with columellate infratectum (with
furcate columellae), spinulate, echinate, microechinate, scabrate, granulate,
psilate, smooth or lophate, often caveate (fenestrate).
Gynoecium Pistil composed of
two (or three) connate carpels. Ovary inferior, unilocular. Style single,
usually bilobate (rarely trilobate), usually with hairs (in, e.g.,
Barnadesioideae glabrous) on which pollen grains are deposited
(secondary pollen display), occasionally hollow. Stigmatic surfaces present on
adaxial side of stylar branches (inner surface of stylar branches stigmatic),
papillate, Dry type; apex of stylar branches rounded, obtuse or with different
appendages; base of stylar branches often inflated. Male flowers usually with
pistillodium.
Ovules Placentation basal.
Ovule one per ovary, anatropous, ascending, epitropous, unitegmic,
tenuinucellar. Integument six to twenty cell layers thick, rarely vascularized.
Archespore usually unicellular (sometimes multicellular, rarely bicellular).
Megagametophyte usually monosporous, Polygonum type (sometimes
disporous, Allium type, rarely tetrasporous, Chrysanthemum
type?, or unspecified). Synergids often elongated (in, e.g.,
Arctotideae and Calenduleae synergid haustorium present, i.e.
synergids sometimes haustorial). Antipodal cells often persistent,
proliferating (up to c. 60 cells) and/or multinucleate, and haustorial.
Endosperm development usually cellular (sometimes nuclear or variations).
Endosperm haustoria usually absent. Embryogenesis asterad. Polyembryony and
other types of agamospermy frequent in many lineages.
Fruit Usually an achene,
cypsela, with seed wall adnate to pericarp, and often with persistent and
accrescent calyx and corolla base modified into pappus, often with hairs,
bristles, prickles, hooks or other types of outgrowths (rarely a one-seeded
drupe with fleshy pericarp). Phytomelan layer present on surface of cypsela in
some groups of Asteroideae (Heliantheae).
Seeds Aril absent. Elaiosome
sometimes present. Testa usually vascularized. Exotestal cells thickened,
palisade, flattened or indistinct. Endotesta developed into endothelium,
integumentary tapetum. Perisperm not developed. Endosperm thin, oily and
proteinaceous, or absent. Embryo large, straight, oily, without chlorophyll.
Cotyledons usually two (possibly absent in, e.g., Syneilesis).
Germination phanerocotylar.
Cytology x = usually 9, 10,
12, 17, or 18 (lowest n = 2, highest n = 120) – Polyploidy, aneuploidy and
agamospermy frequently occurring. Protein bodies present in nucleus.
DNA Mitochondrial gene
rpl2 absent. “6 bp x 4” inversion present in plastid gene
rbcL. Plastid genome in all Asteraceae except
Barnadesioideae with inversion of 22 kb. Plastid genome in Lactuca
sativa with inversion of 4 kb and, possibly, additional shorter
inversion.
Phytochemistry Flavonols
(kaempferol, quercetin, quercetagetine based yellow flavonols, etc.),
isoflavonoids, chalcones, cyanidin, coumarins, diterpenoids, terpenoid ethereal
oils and balsams, sesquiterpene lactones (especially in latex; responsible for
bitter taste of numerous Asteraceae), ursolic acid,
caffeic acid, verbascosides (rare), pentacyclic triterpene alcohols, ellagic
acid (rare), caurane and other alkaloids, triterpene saponins,
phenylalanine-derived cyanogenic compounds (rare), lignans, fatty acids (in
seeds), fatty acid derived polyacetylenes (heterocyclic, aromatic or with
vinylic terminal groups; above all in resinous ducts), arbutin, polyacetate
derived arthroquinones, chlorogenic and isochlorogenic acids, and steroids
present. Pyrrolizidine alkaloids as macrocyclic aliphatic monocarboxylic
diesters (seneciphylline, retrorsine, senecionione, etc.), as well as
furanoeremophilane sesquiterpenes abundant in Senecioneae.
Carbohydrates stored as oligo- or polyfructosans (e.g. inulin) with isokestose
bonds (fructans with unbranched chain); starch absent. Selenium accumulated in
some species. Iridoids, tannins, and acetylene compounds not found.
Use Ornamental plants, spices
(Artemisia dracunculus), vegetables (Cynara, Helianthus
tuberosus, Scorzonera hispanica, Tragopogon porrifolius,
Cichorium intybus var. endive, Lactuca sativa,
Acmella oleracea, Smallanthus sonchifolius), seed oils
(Helianthus annuus, Carthamus tinctorius, Guizotia
abyssinica etc.), medicinal plants, insecticides (Tanacetum
cinerariifolium, Pyrethrum), cosmetics, perfumes, dyeing
substances (Carthamus tinctorius etc.), rubber (Taraxacum
bicorne, Parthenium argentatum), timber.
Systematics Asteraceae are sister to Calyceraceae.
Asteraceae share a
paleotetraploid ancestor with Calyceraceae (Barker & al.
2016). A paleohexaploidization occurred at a very early stage of the evolution
of Asteraceae.
A probable topology of Asteraceae is the following:
[Barnadesioideae+[Famatinanthoideae+[Mutisioideae+Stifftioideae+[Wunderlichioideae+[Gochnatioideae+[Hecastocleidodoideae+[Carduoideae+[Pertyoideae+[Gymnarrhenoideae+[Cichorioideae+[Corymbioideae+Asteroideae]]]]]]]]]]].
Barnadesioideae (D.
Don) K. Bremer et R. K. Jansen in Ann. Missouri Bot. Gard. 79: 415. 6 Mai
1992
9/c 96. Schlechtendalia (1;
S. luzulifolia; southern Brazil, Uruguay, northern Argentina),
Doniophyton (2; D. anomalum, D. weddellii; the Andes
of Chile and Argentina, Patagonia), Duseniella (1; D.
patagonica; Patagonia in southern Argentina), Fulcaldea (1;
F. laurifolia; the Andes in Ecuador and Peru), Barnadesia
(23; Colombia to northern Argentina, especially in the Andes), Huarpea
(1; H. andina; the Andes in San Juan in Argentina),
Dasyphyllum (c 40; South America, especially in the Andes),
Arnaldoa (4; A. argentea, A. coccinosantha, A.
macbrideana, A. weberbaueri; the Andes in Ecuador and Peru),
Chuquiraga (23; the Andes in Colombia to Chile, Patagonia). –
Southern South America, Brazil, with their highest diversity in the Andes.
Trees, shrubs or herbs. Leaves alternate (spiral), opposite or verticillate.
Axillary spines frequent. Capitula discoid, radiate or ligulate. Involucral
bracts chartaceous. Corolla tubular, split, ligulate or labiate (usually with
upper lip quadrilobate and lower lip simple; rarely with upper lip trilobate
and lower lip bilobate). Petal with midvein. Corolla and cypsela bristles with
long tricellular hairs (epidermal cell indistinct, basal cell short and
thick-walled, third cell elongate and thin-walled). Corolla in
Arnaldoa (4+1)-bilabiate. Anther thecae calcarate or ecalcarate and
caudate or ecaudate. Pollen grains prolate, spinulate, microechinate, scabrate,
granulate, or smooth (rarely lophate and not spinulate), with intercolpar
depressions (caveate, with cavity between columellae and foot layer). Style
glabrous or papillate below branching point, bilobate, with stylar branches
short, widened at apex. Stigma lobate. Cypsela densely villose with typical
trichomes. Pappus uniserate, plumose, barbellate, or setaceous (rarely
glabrous). x = 9. Flavonoids sparsely present. Flavones absent.
[Famatinanthoideae+[Mutisioideae+Stifftioideae+[Wunderlichioideae+[Gochnatioideae+[Hecastocleidoideae+[Carduoideae+[Pertyoideae+[Gymnarrhenoideae+[Cichorioideae+[Corymbioideae+Asteroideae]]]]]]]]]]
Corolla usually zygomorphic. Style with
rigid sweeping pollen-collecting hairs and often long branches. Cypsela with
twin hairs (with unicellular to uniseriate base, apical cell simple or equally
bifid). Pappus developing late, usually consisting of capillary bristles
(sometimes scales, awns etc.). n = 2 to more than 100. Inversion of 22,8 kb in
plastid DNA with internal inversion of 3,3 kb.
Famatinanthoideae S.
E. Freire, Ariza et Panero in Mol. Phylogen. Evol. 80: 49. Nov 2014
1/1. Famatinanthus (1; F.
decussatus; the Andes in northwestern Argentina). – Style with
cobblestone-shaped surface, comprising epidermal cells often with periclinal
walls; stylar hairs absent. n = 27 (paleohexaploid). – Panero & al.
(2014) found Famatinanthus to be sister to all Asteraceae except
Barnadesioideae.
[Mutisioideae+Stifftioideae+[Wunderlichioideae+[Gochnatioideae+[Hecastocleidoideae+[Carduoideae+[Pertyoideae+[Gymnarrhenoideae+[Cichorioideae+[Corymbioideae+Asteroideae]]]]]]]]]
Mutisioideae (Cass.)
Lindl. in Loud., Encycl. Plant.: 1074. 1829
45/630–635. South America, Africa,
Asia. Usually perennial herbs (sometimes shrubs). Leaves alternate. Pollen
grains prolate to spheroidal, with psilate exine. Style bilobate, branches
rounded at apex. n = (6–)9 or more; x = 9.
Onoserideae Solbrig in
Taxon 12: 231. Jul 1963
6/42. Aphyllocladus (4; A.
denticulatus, A. ephedroides, A. sanmartinianus, A.
spartioides; the Andes in southern Bolivia, northern Chile and
northwestern Argentina), Gypothamnium (1; G. pinifolium; the
Atacama Desert in northern Chile), Lycoseris (11; Central America,
tropical South America), Onoseris (21; Mexico, Central America, the
Andes in tropical South America), Plazia (4; P.
cheiranthifolia, P. conferta, P. daphnoides, P.
robinsonii; the Andes from Colombia to Chile and Argentina),
Urmenetea (1; U. atacamensis; the Andes in northern Chile and
northwestern Argentina). – Tropical America, the Andes. Shrubs or herbs.
Capitula radiate, with imbricate involucral bracts. Ray corollas bilabiate,
disc corollas quinquelobate tubular. Anther thecae calcarate and caudate.
Pappus consisting of bi- to quadriseriate bristles.
[Mutisieae+Nassauvieae]
Mutisieae Cass. in J.
Phys. Chim. Hist. Nat. Arts 88: 199. Mar 1819
15/c 280. Lulia (1; L.
nervosa; Brazil), Brachyclados (3; B. caespitosus,
B. lycioides, B. megalanthus; Chile, Argentina),
Adenocaulon (5; A. nepalense: Nepal; A. himalaicum:
northern India to China, the Korean Peninsula, Japan and eastern Siberia;
A. bicolor: southern Canada, United States; A. lyratum:
Mexico, Guatemala; A. chilense: Chile, Argentina),
Trichocline (24; western and southern South America, one species,
T. spathulata, in southwestern Western Australia),
Chaetanthera (c 50; southern Peru, Chile, the Andes in Argentina),
Amblysperma (2; A. scapigera, A. spathulata;
southwestern Western Australia), Chaptalia (c 70; southern United
States, Mexico, Central America, the West Indies, tropical South America),
Chucoa (2; C. ilicifolia, C. lanceolata; the Andes
in Peru and Bolivia), Eriachaenium (1; E. magellanicum;
Patagonia in southern Argentina), Gerbera (c 40; northeastern,
tropical and southern Africa, Madagascar, southern Asia to China),
Oreoseris (12; eastern Turkey, Armenia, Central Asia, the Himalayas,
China, northern Thailand and Vietnam), Leibnitzia (7; L.
anandria, L. cryptogama, L. knorringiana, L.
lyrata, L. occimadrensis, L. phanerogama, L.
pusilla; southern and eastern Asia, Arizona, New Mexico, northern Mexico),
Mutisia (c 60; the Andes in Colombia to southern Chile and Argentina,
southeastern Brazil, Uruguay, Paraguay, northeastern Argentina),
Pachylaena (2; P. atriplicifolia, P. rosea; the
Andes in Chile and Argentina), Perdicium (2; P. capense,
P. leiocarpum; Western Cape). – Africa, Madagascar, Armenia,
southern and Central Asia to China, southwestern Australia, America, with their
highest diversity in South America. Usually herbs (sometimes shrubs). Capitula
usually radiate (sometimes di sciform or discoid), with imbricate involucral
bracts. Ray corollas tridentate or bilabiate (rarely tubular) strap-shaped or
absent, disc corollas bilabiate or tubular, shallowly lobate. Anther thecae
usually ecalcarate and caudate. Style shallowly bilobate, with long papillate
branches (papillae rounded). Cypsela often glandular Pappus consisting of uni-
or multiseriate scabrid to plumose usually capillary bristles, or absent.
Nassauvieae Cass. in
J. Phys. Chim. Hist. Nat. Arts 88: 198. Mar 1819
24/310–315. Moscharia (2;
M. pinnatifida, M. solbrigii; central Chile),
Oxyphyllum (1; O. ulicinum; northern Chile),
Polyachyrus (7; P. annuus, P. carduoides, P.
cinereus, P. fuscus, P. gayi, P. oblongiflorus,
P. sphaerocephalus; Peru to the Andean slopes in central Chile),
Leucheria (47; the Andes from southern Peru to Chile and Patagonia,
the Falkland Islands), Spinoliva (1; S. ilicifolia; Chile,
Argentina), Dolichlasium (1; D. lagascae; the central Andes
in Argentina), Jungia (c 30; Mexico, Central America, the Andes to
northern Chile and northwestern Argentina), Trixis (c 40; southwestern
United States, Mexico, Central America, the West Indies, tropical South
America), Berylsimpsonia (2; B. crassinervis, B.
vanillosma; the Greater Antilles), Acourtia (c 85; southwestern
United States, Mexico, Central America, the West Indies), Holocheilus
(7; H. brasiliensis, H. fabrisii, H. hieracioides,
H. illustris, H. monocephalus, H. pinnatifidus,
H. schulzii; southern Brazil, Uruguay, Paraguay, northern and central
Argentina), ‘Perezia’ (36; the Andes from Colombia to southern
Patagonia, southern Brazil, Uruguay, Paraguay, northeastern Argentina;
non-monophyletic), Nassauvia (c 40; the southern Andes in Bolivia,
Chile and Argentina, Patagonia, the Falkland Islands; incl.
Calopappus?), Calopappus (1; C. acerosus; the Andes
in central Chile; in Nassauvia?), Proustia (2; P.
cuneifolia, P. pyrifolia; the Andes from southern Peru to
Bolivia, Chile and Argentina); unplaced: Ameghinoa (1; A.
patagonica; Patagonia in Argentina), Burkartia (1; B.
lanigera; Patagonia), Cephalopappus (1; C. sonchifolius;
northeastern Brazil), Criscia (1; C. stricta; Brazil,
Uruguay, northern Argentina), Leunisia (1; L. laeta; the
Andes in central Chile), Macrachaenium (1; M. gracile; the
Andes, Patagonia, Tierra del Fuego), Marticorenia (1; M.
foliosa; central Chile), Pamphalea (2; P. bupleurifolia,
P. cardaminifolia; Brazil, Uruguay, Argentina), Pleocarphus
(1; P. revolutus; northern Chile). – Tropical America, subtropical
and temperate South America, with their largest diversity in the Andes. Shrubs,
lianas or herbs. Capitula discoid or radiate, with imbricate involucral bracts.
Ray corollas bilabiate, disc corollas usually bilabiate. Anther thecae
calcarate and caudate. Stylar branches with apical crown of sweeping hairs.
Pappus consisting of uni- to multiseriate paleaceous to plumose capillary
bristles.
Stifftioideae (D. Don)
Panero in Panero et Funk in Phytologia 89: 356. Dec 2007
9/41. Stifftia
(6; S. cayennensis, S. chrysantha, S. fruticosa,
S. hatschbachii, S. parviflora, S. uniflora;
northeastern Brazil, French Guiana); Achnopogon (2; A.
steyermarkii, A. virgatus; Venezuela), Duidaea (4;
D. marahuacensis, D. pinifolia, D. rubriceps, D.
tatei; Venezuela, Guyana), Glossarion (2; G. bilabiatum,
G. rhodanthum; Venezuela, Guyana), Gongylolepis (15; tropical
South America, with their largest diversity in Venezuela and the Guayana
Highlands), Neblinaea (1; N. promontorium; Venezuela,
Guyana), Quelchia (4; Q. bracteata, Q. cardonae,
Q. conferta, Q. eriocaulis; Venezuela, Guyana),
Salcedoa (1; S. mirabaliarum; Hispaniola);
Hyaloseris (6; H. andrade-limae, H. camataquiensis,
H. cinerea, H. longicephala, H. quadriflora, H.
rubicunda; Bolivia, Argentina). – Hispaniola, South America, with their
largest diversity in the Venezuela and the Guayana Highlands. Trees or shrubs
(sometimes lianas). Leaves alternate or opposite. Capitula discoid or ligulate,
with multiseriate imbricate involucral bracts. Corolla actinomorphic and
quinquelobate (Stifftia)
or zygomorphic and usually (3+2)-bilabiate with coiled lobes
(Gongylolepis clade) or actinomorphic to zygomorphic and
(4+1)-subbilabiate to quinquedentate ligulate (Hyaloseris clade).
Anther thecae calcarate and caudate. Pollen grains usually prolate, echinate or
psilate. Style bilobate, with rounded to shortly acute apex. Pappus showy,
consisting of several series of numerous scabrid capillary bristles or setae. x
= 9. – A probable topology is [Stifftia+[Gongylolepis
clade+Hyaloseris clade]]. Stifftioideae appear to be
sister-group to the remaining Asteraceae (Panero & al.
2014).
[Wunderlichioideae+[Gochnatioideae+[Hecastocleidoideae+[Carduoideae+[Pertyoideae+[Gymnarrhenoideae+[Cichorioideae+[Corymbioideae+Asteroideae]]]]]]]]
Wunderlichioideae
Panero et V. A. Funk in Phytologia 89: 357. Dec 2007
8/44. The Venezuelan and the Guayana
Highlands, eastern South America, southwestern China. Style and stylar branches
glabrous. x = 9. Deletion in plastid gene rpoB.
Wunderlichieae Panero
et V. A. Funk in Phytologia 91: 570. 1 Dec 2009
4/38. Wunderlichia (6; W.
azulensis, W. bahiensis, W. cruelsiana, W.
insignis, W. mirabilis, W. senaeii; Brazil);
Chimantaea (9; the Pantepui area of the Guayana Highlands in
Venezuela, Guyana and northern Brazil), Stenopadus (17; the Guayana
Highlands), Stomatochaeta (6; S. acuminata, S.
condensata, S. crassifolia, S. cylindrica, S.
cymbifolia, S. steyermarkii; the Guayana Highlands in Venezuela,
Guyana and northern Brazil). – Northeastern tropical South America. Trees or
shrubs. Capitula discoid, with imbricate involucral bracts. Corolla
actinomorphic, deeply quinquelobate. Anther thecae calcarate and caudate.
Pappus consisting of multiseriate bristles or setae.
Hyalideae Panero in J.
L. Panero et V. A. Funk in Phytologia 89: 358. Dec 2007
4/6. Ianthopappus (1; I.
corymbosus; southern Brazil, Uruguay, northern Argentina), Hyalis
(2; H. argentea, H. lancifolia; southern Bolivia, Paraguay,
Argentina); Leucomeris (2; L. decora, L.
spectabilis; the Himalayas, Yunnan, Burma, Thailand, Vietnam),
Nouelia (1; N. insignis; southwestern China). – The
Himalayas to southwestern China and Vietnam, South America. Shrubs or small
trees. Capitula radiate or discoid, with imbricate involucral bracts. Ray
corollas bilabiate (with coiled lobes) or absent, disc corollas tubular, deeply
quinquelobate. Anther thecae calcarate and caudate. Pappus consisting of bi- or
triseriate scabrid to smooth capillary bristles.
[Gochnatioideae+[Hecastocleidoideae+[Carduoideae+[Pertyoideae+[Gymnarrhenoideae+[Cichorioideae+[Corymbioideae+Asteroideae]]]]]]]
Gochnatioideae Panero
et V. A. Funk in Proc. Biol. Soc. Washington 115: 913. 30 Dec 2002
8/90. Cyclolepis (1; C.
genistoides; Paraguay to northern Patagonia in Argentina);
Gochnatia (8; the central Andes), Pentaphorus (2; P.
foliolosus, P. rosmarinifolius; the southern Andes in Chile and
Argentina), Nahuatlea (7; N. arborescens, N.
hiriartiana, N. hypoleuca, N. magna, N.
obtusata, N. purpusii, N. smithii; southern Arizona and
Texas, Mexico), Anastraphia (33; Mexico, Cuba, Hispaniola, the
Bahamas), Cnicothamnus (2; Bolivia, northwestern Argentina),
Moquiniastrum (21; tropical South America, with their highest
diversity in Brazil), Richterago (16; southeastern Brazil, Uruguay,
northeastern Argentina), Cnicothamnus (2; C. azafrin, C.
lorentzii; Bolivia, Paraguay, northwestern Argentina). – Mexico, Central
America, the Greater Antilles, South America. Trees, shrubs or perennial herbs.
Capitula radiate or discoid, with tri- to decemseriate imbricate involucral
bracts. Ray corollas bilabiate or absent, disc corollas deeply quinquelobate
tubular. Anther thecae ecalcarate and caudate. Pollen prolate to spheroidal,
anthemoid type. Stylar branches usually short and glabrous, with rounded
apices. Cypsela villose. Pappus consisting of uni- to triseriate scabrid or
plumose capillary (or sometimes flat) bristles. n = 22, 23, 27; x = 9. –
Cyclolepis genistoides is often recovered as sister to the remaining
Gochnatioideae (Mandel & al. 2017) or, sometimes, as sister to
Wunderlichieae (Funk & al. 2014).
[Hecastocleioideae+[Carduoideae+[Pertyoideae+[Gymnarrhenoideae+[Cichorioideae+[Corymbioideae+Asteroideae]]]]]]
Pollen grains spheroidal. Columellae
sausage-like. Deletion in plastid gene rpoB.
Hecastocleidoideae
Panero et V. A. Funk in Proc. Biol. Soc. Washington 115: 914. 30 Dec 2002
1/1. Hecastocleis (1; H.
shockleyi; southern Nevada and adjacent California). – Shrub. Capitula
discoid, single-flowered, with imbricate involucral bracts. Corolla
actinomorphic, deeply quinquelobate tubular. Anther thecae calcarate and
caudate. Pollen grains spheroidal, tricolpate. Stylar branches short, with
rounded apex. Pappus consisting of six scales (sometimes fused). x = 8.
[Carduoideae+[Pertyoideae+[Gymnarrhenoideae+[Cichorioideae+[Corymbioideae+Asteroideae]]]]]
Carpels of disc florets superposed (one
above the other). Style with pollen collecting hairs (sweeping hairs). x = 10.
Deletion and insertion in plastid gene rpoB.
Carduoideae Cass. ex
Sweet, Hort. Brit.: 213. Jul-Aug 1826
89/3.160–3.285. Cosmopolitan, although
mainly in the Northern Hemisphere (especially Eurasia and North Africa). Leaves
alternate (spiral), often spinose-serrate. Flowers usually actinomorphic.
Pollen grains spheroidal, sometimes psilate, with internal tectum, sometimes
with internal foramina. Columellae medium thickness. Style with hair collar
below branches. Cypsela usually with twin hairs. Exotestal cells palisade. x =
9–11. – Probable topology is
[Cardueae+Dicomeae+[Oldenburgieae+Tarchonantheae]].
Cardueae Cass. in J.
Phys. Chim. Hist. Nat. Arts 88: 155. Feb 1819 [‘Carduineae’]
78/3.020–3.145. Cardopatium
(1; C. corymbosum; eastern Mediterranean to Turkey),
Cousiniopsis (1; C. atractyloides; Central Asia),
Thevenotia (2; T. persica, T. scabra; Iran,
Afghanistan, Central Asia), Tugarinovia (1; T. mongolica;
Mongolia, Inner Mongolia), Atractylodes
(8–11; India, Burma, Vietnam, China, the Korean Peninsula, Japan, the Russian
Far East), Atractylis
(14–30; southern Europe, the Canary Islands, the Mediterranean, North Africa,
temperate Asia), Carlina
(c 35; Europe, Macaronesia, the Mediterranean, western Asia), Echinops
(190–195; Europe, the Mediterranean to Central Asia, tropical African
mountains), Dipterocome (1; D. pusilla; eastern Mediterranean
to Afghanistan), Amphoricarpos (5; A. autariatus, A.
elegans, A. exsul, A. neumayerianus, A.
praedictus; the Balkan Peninsula, Greece and Turkey to the Caucasus),
Chardinia (1; C. orientalis; western Asia), Xeranthemum
(5; X. annuum, X. cylindraceum, X. inapertum, X.
longepapposum, X. squarrosum; the Mediterranean, southwestern
Asia), Siebera (2; S. nana, S. pungens; southwestern
Asia to Afghanistan), Berardia
(1; B. subacaulis; western Alps), Staehelina
(3; S. dubia, S. petiolata, S. unifloscula; the
Mediterranean), Hirtellina (2; H. fruticosa, H.
lobelii; warm-temperate regions in southeastern Europe and Asia),
Shangwua (3; S. denticulata, S. jacea, S.
masarica; Tajikistan, northern Pakistan, Pamir, Tibet, the Himalayas,
Yunnan), Onopordum
(c 45; Europe, the Mediterranean, western Asia), Xanthopappus (1;
X. subacaulis; Mongolia, northwestern China), Ancathia (1;
A. igniaria; Central Asia, Mongolia, China), Synurus (3;
S. deltoides, S. excelsus, S. pungens; East Asia),
Alfredia (7; A. acantholepis, A. aspera, A.
cernua, A. fetissowii, A. integrifolia, A.
nivea; Central to East Asia), Lamyropappus (1; L.
shacaptaricus; Central Asia), Syreitschikovia (3; S.
spinulosa, S. tenuifolia, S. tenuis; Central Asia),
Olgaea (18; Central Asia to northern China), Cynara
(11; the Canary Islands, the Mediterranean), Ptilostemon
(18; the Mediterranean), Galactites
(4; G. duriaei, G. mutabilis, G. rigualii, G.
tomentosa; the Canary Islands, the Mediterranean), Lamyropsis
(5; L. charadzeae, L. cynaroides, L. macracantha,
L. microcephala, L. sinuata; the Mediterranean, southwestern
Asia), Notobasis
(1; N. syriaca; the Mediterranean to Central Asia), Picnomon
(1; P. acarna; the Mediterranean, southwestern Asia), Silybum
(2; S. eburneum, S. marianum; the Mediterranean), ‘Carduus’
(c 115; Europe, Macaronesia, the Mediterranean, East African mountains,
temperate Asia; paraphyletic), Cirsium
(c 465?; temperate regions on the Northern Hemisphere, the Mediterranean), Tyrimnus
(1; T. leucographus; the Mediterranean), Arcyna (1; A.
tournefortii; the Iberian Peninsula), Takeikadzuchia (1; T.
lomonosowii; Mongolia, northern China), Arctium
(14; temperate regions in the Old World), Cousinia (680–690; eastern
Mediterranean to Central Asia and western Himalayas), Schmalhausenia
(1; S. nidulans; Tienshan), Hypacanthium (2; H.
echinopifolium, H. evidens; Central Asia), Saussurea
(430–440; Europe, temperate Asia, with their largest diversity in the
Himalayas, Tibet and western China), Himalaiella (13; the Himalayas),
Jurinea
(200–210; Central and South Europe, the Mediterranean, northwestern Africa,
southwestern and Central Asia, with their largest diversity in Central Asia),
Goniocaulon (1; G. glabrum; tropical Africa, India),
Schischkinia (1; S. albispina; southwestern to Central Asia),
Volutaria
(c 16; Macaronesia, the Mediterranean, southwestern Asia, tropical East
Africa), Cheirolophus
(c 25; southwestern Europe, Madeira, the Canary Islands, North Africa),
Myopordon (5; M. aucheri, M. hyrcanum, M.
persicum, M. pulchellum, M. thiebautii; southwestern to
Central Asia), Rhaponticum
(27; Tenerife, the Mediterranean, temperate and subtropical Asia, eastern and
southeastern Australia), Callicephalus (1; C. nitens;
southwestern to Central Asia), Mantisalca
(4; M. amberboides, M. delestrei, M. duriaei, M.
salmantica; the Mediterranean, North Africa), Centaurothamnus (1;
C. maximus; the Arabian Peninsula), Ochrocephala (1; O.
imatongensis; Ethiopia), Serratula
(2; S. coronata, S. tinctoria; temperate Eurasia to China),
Klasea (c 55; temperate Eurasia, North Africa), Stizolophus
(3; S. balsamita, S. balsamitoides, S.
coronopifolius; Turkey to the Caucasus, Armenia, the Near East, Iran),
Centaurodendron (2; C. dracaenoides, C. palmiforme;
Juan Fernandez Islands), Plectocephalus (7; P. americanus,
P. cachinalensis, P. chilensis, P. floccosus, P.
rothrockii, P. tweediei; United States, Mexico, tropical South
America, Chile, one species, P. varians, in Ethiopia), Zoegea
(3; Z. crinita, Z. leptaurea, Z. purpurea; Egypt,
Turkey and the Arabian Peninsula to Central Asia and India), Psephellus
(85–90; eastern Mediterranean, southwestern to Central Asia, southern
Siberia, one species, P. sibiricus, in China), Rhaponticoides
(23; Europe, the Mediterranean, temperate Asia), ’Centaurea’
(350–400?; Europe, the Mediterranean, tropical African mountains,
southwestern and northern Asia; paraphyletic), Carthamus
(45–50; the Mediterranean to Central Asia; incl. Carduncellus
and Femeniasia?),
Carduncellus
(4; C. coeruleus, C. hispanicus, C. mairei, C.
monspeliensis; western and central Mediterranean; in Carthamus?),
Femeniasia
(1; F. balearica; northwestern Menorca; in Carthamus?). –
Unplaced Cardueae Dolomiaea
(15; the Himalayas, Tibet), Diplazoptilon (2; D. cooperi,
D. picridifolium; southwestern China), Polytaxis (3; P.
lehmannii, P. pulchella, P. winkleri; Central Asia), Crupina
(5; C. crupinastrum, C. intermedia, C.
pseudocrupina, C. strum, C. vulgaris; the Mediterranean,
southwestern Asia to China), Amberboa (9; the Mediterranean to Central
Asia), Tricholepis (17; Central Asia to Burma), Plagiobasis
(1; P. centaureoides; Central Asia), Russowia (1; R.
sogdiana; Turkestan), Karvandarina (1; K. aphylla; Iran,
Pakistan), Oligochaeta (4; O. divaricata, O. minima,
O. ramosa, O. tomentosa; southwestern to Central Asia,
India), Cavea (1; C. tanguensis; eastern Himalayas),
Pilostemon (2; P. filifolia, P. karategini; Central
Asia to China). – Temperate, arid subtropical and alpine regions in the Old
World, with their highest diversity in the Mediterranean and eastwards to
Central Asia. Usually herbs (sometimes shrubs, rarely trees). Roots with
laticifers. Capitula discoid, with multiseriate involucral bracts (often
spiny). Corolla usually actinomorphic, deeply quinquelobate, usually tubular,
straight or s-shaped. Anther thecae calcarate and caudate. Pollen grains
sometimes with tectum echinate. Style with short hairs above branching point,
below with papillose-pilose thickened articulation (“pollen brush”).
Cypsela walls with phytomelan. Pappus consisting of scales or bristles. x =
10.
Dicomeae Panero et V.
A. Funk in Proc. Biol. Soc. Wash. 115: 916. 20 Dec 2002
8/c 113. ‘Pasaccardoa’ (4;
P. baumii, P. grantii, P. jeffreyi, P.
procumbens; tropical and southern Africa; non-monophyletic),
Dicomopsis (1; D. welwitschii; Angola), Dicoma (36;
tropical and southern Africa, Madagascar, one species, D. chatanensis,
on the Arabian Peninsula and Socotra, one species, D. tomentosa, in
Pakistan and India), Cloiselia (2; Madagascar), Macledium (c
20; tropical and southern Africa); Erythrocephalum (15; tropical East
Africa), Pleiotaxis (c 35; tropical and southern Africa),
Gladiopappus (1; G. vernonioides; Madagascar). – Tropical
and southern Africa, Madagascar, Socotra, the Arabian Peninsula, Pakistan,
India. Small trees, shrubs or perennial herbs. Capitula discoid or radiate,
with imbricate involucral bracts, often with hyaline margins. Ray corollas
bilabiate, disc corollas deeply quinquelobate, tubular. Anther thecae calcarate
and caudate. Stylar branches with apical acute sweeping hairs. Cypsela with
twin hairs. Pappus consisting of scabrid to plumose multiseriate bristles or
scales. x = 11. – Erythrocephalum, Pleiotaxis and
Gladiopappus differ from the rest and are provisionally included in
Dicomeae.
[Oldenburgieae+Tarchonantheae]
Oldenburgieae S.
Ortiz, Compositae Newslett. 47: 2. 15 Apr 2009
1/4. Oldenburgia (4; O.
grandis, O. intermedia, O. papionum, O.
paradoxa; southern parts of Western and Eastern Cape). – Small trees or
shrubs. Capitula radiate, with imbricate involucral bracts, with or without
hyaline margins. Ray corollas bilabiate, disc corollas usually actinomorphic,
deeply quinquelobulate, tubular. Anther thecae calcarate and caudate. Pollen
grains with tectum echinate. Stylar branches usually without sweeping hairs.
Pappus consisting of scabrid to plumose multiseriate bristles. x = 9.
Tarchonantheae Vis.,
Fl. Dalmat. 2: 24, 60. 10 Nov 1847
2/20. Brachylaena (14; tropical
and southern Africa, Madagascar, the Mascarene Islands), Tarchonanthus
(6; T. camphoratus, T. littoralis, T. minor, T.
obovatus, T. parvicapitulatus, T. trilobus; Africa, the
Arabian Peninsula). – Tropical and southern Africa, Madagascar, the Mascarene
Islands, the Arabian Peninsula. Trees or shrubs. Capitula discoid, with
imbricate involucral bracts, with or without hyaline margins. Corolla of male
florets actinomorphic, deeply quinquelobate, tubular. Corolla of female florets
actinomorphic, tri- to quinquelobate, tubular to filiform. Anther thecae
calcarate and caudate. Stylar branches without sweeping hairs. Pappus
consisting of uni- or biseriate barbellate bristles (sometimes absent). x =
9.
[Pertyoideae+[Gymnarrhenoideae+[Cichorioideae+[Corymbioideae+Asteroideae]]]]
Pollen grains with unbranched
columellae.
Pertyoideae Panero et
V. A. Funk in Proc. Biol. Soc. Washington 115: 915. 30 Dec 2002
4/95–100. Pertya
(c 25; Afghanistan, China, Japan, Thailand), Ainsliaea (c 70;
Afghanistan, the Himalayas, Bangladesh, East Asia to Japan, Southeast Asia,
West Malesia to the Philippines), Myripnois (1; M. dioica;
northern China), Catamixis (1; C. baccharoides; northern
India, western Nepal). – Afghanistan, the Himalayas to Japan and Southeast
Asia, West Malesia to the Philippines. Corolla deeply but unequally divided.
Pollen grains spheroidal. Stylar branches “short”, hairy to papillate on
abaxial side, with apex rounded to acuminate. Pappus consisting of bristles
(sometimes plumose). x = 13, 14. – The position of Catamixis (1) is
unresolved. Pertyoideae resemble Mutisieae but the flowers
are not bilabiate.
[Gymnarrhenoideae+[Cichorioideae+[Corymbioideae+Asteroideae]]]
Pollen grains with tectum echinate.
Gymnarrhenoideae
Panero et V. A. Funk in Proc. Biol. Soc. Washington 115: 912. 30 Dec 2002
1/1. Gymnarrhena (1; G.
micrantha; North Africa, southwestern Asia). – Amphicarpic annuals.
Capitula aggregated into synflorescences, with florets surrounded by small
involucral bracts. Synflorescences disciform, congested in centre of leaf
rosette. Corolla in subterranean capitula vestigial, in aerial capitula
filiform. Anther thecae ecalcarate and caudate. Pollen grains spheroidal;
colpus ends acute; tectum echinate, non-lophate. Stylar branches long. Cypsela
of three types, with twin hairs. Pappus consisting of scale-like bristles, or
vestigial to absent. x = 10. – Subterranean capitula are homogamous, with
entirely female florets, and cleistogamous. Aerial capitula are heterogamous,
functionally male florets interspersed with female florets.
[Cichorioideae+[Corymbioideae+Asteroideae]]
Pollen grains at least sometimes caveate; tectal
spines 2–5 µm. Stylar branches “medium” to “long”. Deletion in
plastid gene ndhF.
Cichorioideae (Juss.)
Chevall., Fl. Gén. Env. Paris 2: 531. 5 Jan 1828
280/11.270–>16.300. Cosmopolitan.
Ray florets present in some species, disc florets strap-shaped and deeply
lobate. Pollen grains spheroidal, sometimes caveate, with cavities separating
columellae from foot layer, or lophate, with internal tectum; columellae
aggregated below spines. Carpels collateral. Stylar branches acute. Hairs
usually acute. x = (7–)9–10(–13). –
[Cichorieae+[Eremothamneae+[Arctotideae+
[Platycarpheae+[Liabeae+[Distephanus+Moquinieae+Vernonieae]]]]]].
– Hetero-lepis (3), shrubs distributed mainly in the Cape provinces,
has sometimes been assigned to Arctotideae, but its systematic
affiliation is not resolved. It has partially connate involucral bracts, and
pappus consisting of biseriate bristle-like scales.
Cichorieae Lam. et
DC., Syn. Plant. Fl. Gall.: 255. 30 Jun 1806 [‘Cichoraceae’]
107/9.500–>14.650.
Warioniinae Gemeinholzer et H. Kilian in V. A. Funk
et al., Syst. Evol. Biogeogr. Compositae: 380. 25 Aug 2009. Warionia
(1; W. saharae; northwestern Sahara). –
Scorzonerinae Dumort., Fl. Belg.: 63. 1827
[‘Scorzonereae’]. Tourneuxia (1; T. variifolia;
Morocco to Libya), Lasiospora (2; L. eriolaena, L.
hirsuta; the Mediterranean to Greece), Scorzonera (180–200;
Europe, the Mediterranean to western and Central Asia),
Takhtajaniantha (1; T. pusilla; Armenia and Iran to Central
Asia, Pakistan, Mongolia, the Arabian Peninsula), Geropogon (1; G.
hybridus; southern Europe, the Mediterranean, Turkey to Iran),
Podospermum (c 20; the Mediterranean to Central Asia, North Africa),
Epilasia (3; E. acrolasia, E. hemilasia, E.
mirabilis; the Middle East, western and Central Asia to northwestern
China), Tragopogon (c 110; Europe, the Mediterranean, temperate Asia,
southern Canada, United States), Koelpinia (5–7; K.
chrysoglochis, K. deflexa, K. linearis, K.
macrantha, K. sessilis, K. tenuissima, K.
turanica; the Mediterranean, North Africa, Iraq to Central Asia),
Pterachaenia (1; P. stewartii; Afghanistan, Pakistan). –
Scolyminae Less., Syn. Gen. Compos.: 126. Jul–Aug
1832 [‘Scolymeae’]. Gundelia (1–9; G.
tournefortii; Cyprus, Turkey to Afghanistan), Catananche (5;
C. arenaria, C. caerulea, C. caespitosa, C.
lutea, C. montana; the Mediterranean to Turkey and Syria, North
Africa), Scolymus (3; S. grandiflorus, S.
hispanicus, S. maculatus; the Mediterranean), Hymenonema
(2; H. graecum, H. laconicum; Greece). –
Hieraciinae Dumort., Fl. Belg.: 62. 1827
[‘Hieracieae’]. Schlagintweitia (3; S.
chamaepicris, S. huteri, S. intybacea; the Pyrenees,
mountains in Central Europe), Andryala (30; the Mediterranean,
Macaronesia, North Africa, the Middle East), Hieracium (>5.000,
probably >10.000; temperate regions on both hemispheres, tropical
mountains), Hispidella (1; H. hispanica; the Iberian
Peninsula), Pilosella (210–220; Europe, the Mediterranean, North
Africa, temperate Asia). – Cichoriinae Dumort.,
Anal. Fam. Plant.: 30. 1829 [‘Cichoreae’]. Phalacroseris
(1; P. bolanderi; Sierra Nevada in California), Erythroseris
(2; E. amabilis: Socotra; E. somalensis: Somalia),
Cichorium (10; Europe, the Mediterranean, Ethiopia),
Rothmaleria (1; R. granatensis; southern Spain),
Tolpis (15–20; southern Europe, Macaronesia, the Mediterranean to
Yemen, North Africa, one species, T. capensis, from Ethiopia to South
Africa and Madagascar, one species, T. mbalensis, in Zambia and
Malawi), Arnoseris (1; A. minima; Europe). –
Microseridinae Stebbins in O. T. Solbrig, Taxon 12:
234. Jul 1963. Krigia (9; central and southern Canada, United States,
northern Mexico), Pinaropappus (7–10; southern United States,
Mexico, Guatemala), Marshalljohnstonia (1; M. gypsophila;
Coahuila in northern Mexico), Shinnersoseris (1; S. rostrata;
southern Canada, central and southern United States, northern Mexico),
Chaetadelpha (1; C. wheeleri; southwestern United States),
Lygodesmia (9; southwestern Canada, United States, Mexico),
Picrosia (2; P. cabreriana, P. longifolia; tropical
and subtropical South America), Pyrrhopappus (1–5; P.
carolinianus, P. grandiflorus, P. pauciflorus, P.
rothrockii, P. taraxacoides; United States, northern Mexico);
Glyptopleura (2; G. marginata, G. setulosa; western
United States), Agoseris (14; western and southern Canada, western
United States, Mexico, Chile, Argentina), Nothocalais (4; N.
alpestris, N. cuspidata, N. nigrescens, N.
troximoides; southwestern Canada, western United States),
Microseris (30–40; southeastern Australia, Tasmania, New Zealand,
western North America, Mexico, Peru, Chile), Stebbinsoseris (2;
California, Arizona, northwestern Mexico; S. decipiens derived from
Microseris bigelovii x Uropappus lindleyi; S.
heterocarpa derived from Microseris douglasii x Uropappus
lindleyi), Uropappus (3; U. kelloggii, U.
lindleyi, U. pruinosus; southwestern Canada, western United
States, northern Mexico), Atrichoseris (1; A. platyphylla;
southwestern United States, northwestern Mexico), Malacothrix (c 20;
western United States, northern Mexico), Munzothamnus (1; M.
blairii; San Clemente Island off California), Stephanomeria (18;
southwestern Canada, western United States, northwestern Mexico),
Rafinesquia (2; R. californica, R. neomexicana;
southwestern United States, northwestern Mexico), Pleiacanthus (1;
P. spinosus; western United States), Prenanthella (1; P.
exigua; western United States, northern Mexico), Anisocoma (1;
A. acaulis; southwestern United States, northwestern Mexico),
Calycoseris (2; C. parryi, C. wrightii; southwestern
United States, northwestern Mexico). – Lactucinae
Dumort., Fl. Belg.: 59. 1827. Notoseris (12; China South of Yangtze),
Cicerbita (c 10; Central Asia, the Himalayas, Yunnan, Thailand,
eastern and central Canada and United States), ‘Lactuca’
(50–75?; nearly cosmopolitan except tropical America; polyphyletic),
Scariola (3; S. albertoregalia, S. amaurophyton,
S. exigua; Afghanistan, Central Asia), Melanoseris (25–30;
the Himalayas, Tibet, China), Cephalorrhynchus (4; C.
chitralensis, C. kossinskyi, C. longifolius, C.
subplumosus; Iran, Central Asia, Pakistan), Mycelis (1; M.
muralis; Europe, Turkey, the Caucasus), Kovalevskiella (3; K.
aitchisoniana, K. kovalevskiana, K. zeravschanica;
Central Asia, Afghanistan), Chaetoseris (c 25; the Himalayas, Tibet,
China), Mulgedium (6; M. cacaliaefolius: the Caucasus; M.
centrale, M. lindheimeri, M. polyanthum: central and
southern United States; M. qinghaicum: China; M. roseum:
Turkestan), Faberia (7; F. cavaleriei, F. ceterach,
F. faberi, F. lancifolia, F. nanchuanensis, F.
sinensis, F. thibetica; Tibet, China), Paraprenanthes
(14; East and Southeast Asia), Parasyncalathium (1; P.
souliei; the Himalayas to western China), Astartoseris (1; A.
triquetra; Cyprus, Lebanon), Stenoseris (5; S.
auriculiformis, S. graciliflora, S. leptantha, S.
tenuis, S. triflora; Central Asia, India, the Himalayas, Tibet,
Burma, China, the Malay Peninsula, Sumatra, Java), Lapsanastrum (4;
L. apogonoides, L. humile, L. takasei, L.
uncinatum; China, the Korean Peninsula, Japan, Taiwan, North America). –
Hyoseridinae Less., Syn. Gen. Compos.: 127. Jul–Aug
1832 [‘Hyoserideae’]. Aposeris (1; A. foetida;
Central Europe), Hyoseris (c 10; the Mediterranean to Turkey, North
Africa), Reichardia (c 10; Macaronesia, the Mediterranean to Syria),
Launaea (c 60; the Canary Islands, the Mediterranean, Africa,
Madagascar, East Asia, the West Indies), ’Sonchus’ (c 140?;
Europe, Macaronesia, the Mediterranean to tropical Africa, Asia to Southeast
Asia, New Zealand; incl. Dendroseris and Thamnoseris). –
Hypochaeridinae Less., Syn. Gen. Compos.: 130.
Jul–Aug 1832 [‘Hypochoerideae’]. Urospermum (2; U.
dalechampii, U. picroides; the Canary Islands, the Mediterranean
to Pakistan), Prenanthes (1; P. purpurea; Europe, the Middle
East), Scorzoneroides (c 25; Europe, the Canary Islands, the
Mediterranean, North Africa to the Middle East, temperate Asia),
Hypochaeris (90–100; Europe, Macaronesia, the Mediterranean, North
Africa, Asia, South America), Helminthotheca (5; H. aculeata,
H. balansae, H. comosa, H. echioides, H.
glomerata; southeastern Europe, the Mediterranean to Iran, North Africa),
Picris (c 60; Europe, the Mediterranean, African mountains, temperate
Asia to northern Australia), Hedypnois (4; H. arenaria,
H. arenicola, H. caspica, H. rhagadioloides;
Macaronesia, the Mediterranean to Iran), Leontodon (c 80; Europe, the
Mediterranean, North Africa, temperate Asia, southwestern Asia to Iran). –
Chondrillinae Lamotte, Cat. Plant. Eur. Centr.: 56.
Jul–Dec 1847 [‘Chondrilleae’]. Phitosia (1; P.
crocifolia; the Taigetos Range in southern Greece), Chondrilla
(25–30; Europe, temperate Asia), Willemetia (2; W.
stipitata: Central and southern Europe; W. tuberosa: the Caucasus
to Iran). – Crepidinae Dumort., Fl. Belg.: 60. 1827
[‘Crepideae’]. Acanthocephalus (2; A.
amplexifolius: Altai; A. benthamianus: Uzbekistan),
Ixeris (25–28; East and Southeast Asia), Taraxacum
(>2.700; temperate regions on the Northern Hemisphere, New Zealand incl.
Stewart Island, Chatham Islands, temperate and subantarctic South America),
Youngia (c 35; Asia to India and Japan), Crepidiastrum (c 25;
East Asia to Japan and Taiwan), Askellia (12; Iran, Central Asia to
Pakistan and Mongolia, northern India, the Himalayas, Tibet, Siberia to the
Russian Far East, China, temperate North America), Crepis (c 200; the
Northern Hemisphere, tropical and southern Africa, South America),
Dianthoseris (1; D. schimperi; East and Northeast African
highlands; in Crepis?), Lagoseris (2; L. ovata,
L. purpurea; Crimea, Turkey), Rhagadiolus (2; R.
edulis: the Mediterranean to Iran; R. stellatus: the Canary
Isles, the British Isles to the Mediterranean, Turkey, the Caucasus),
Lapsana (1; L. communis; Europe, western and southwestern
Asia); Syncalathium (5; S. chrysocephalum, S.
disciforme, S. kawaguchii, S. porphyreum, S.
roseum; Tibet, China), Hololeion (3; H. fauriei:
southern Korean Peninsula; H. krameri: Japan; H.
maximowiczii: China, the Korean Peninsula, the Russian Far East),
Soroseris (7; S. depressa, S. erysimoides, S.
glomerata, S. hookeriana, S. pumila, S. teres,
S. umbrella; the Himalayas, Tibet, western and southwestern China),
Dubyaea (18; the Himalayas, Tibet, western China), Nabalus (c
25; China, the Korean Peninsula, Japan, the Russian Far East, Canada, United
States); Garhadiolus (4; G. hamosus, G. hedypnois,
G. minutissimus, G. papposus; Cyprus, Egypt, Turkey, the
Caucasus, the Middle East and the Arabian Peninsula to Central Asia and China),
Lagoseriopsis (1; L. popovii; Central Asia),
Heteracia (1; H. szovitsii; the Balkan Peninsula, Crimea, the
Caucasus, southwestern and Central Asia to China), Heteroderis (1;
H. pusilla; Egypt, the Arabian Peninsula, southwestern to Central
Asia, Pakistan). – Unplaced Cichorieae
Ixeridium (16; East and Southeast Asia, Malesia to New Guinea),
Spiroseris (1; S. phyllocephala; Pakistan). – Cosmopolitan,
with their highest diversity in temperate regions on the Northern Hemisphere.
Usually herbs (rarely shrubs or small trees). Laticifers (with white latex)
present. Capitula usually ligulate (not in Gundelia and
Warionia), with few outer series of imbricate bracts and one long
inner series of bracts, often with hyaline margin. Corolla quinquedentate
ligulate. Anther thecae calcarate and caudate. Pollen grains echinolophate or
echinate. Style with sweeping hairs. Pappus usually consisting of scales or
scabrid to barbellate or plumose bristles (rarely absent). x = 9. – Several
genera in Cichorieae are agamospermous, i.a. Taraxacum, with
about 2.700 species, and Hieracium, with at least 5.000–5.500 and
probably far more than 10.000 species. In many groups (e.g. in
Pilosella) the species number is constantly in a state of flux, since
the numerous species are not fixed apomicts, but mainly amphiapomictic and
hybridize frequently. – Warionia saharae is sister to the remaining
Cichorieae.
[Eremothamneae+[Arctotideae+[Platycarpheae+[Liabeae+[Distephanus+Moquinieae+Vernonieae]]]]]
Eremothamneae H. Rob.
et Brettell in Phytologia 26: 164. 16 Jul 1973
2/3. Eremothamnus (1; E.
marlothianus; Namibia), Hoplophyllum
(2; H. ferox, H. spinosum; Northern and Western Cape). –
Western South Africa, southern Namibia. Shrubs. Capitula radiate or discoid,
with gradate multiseriate involucral bracts, distally papyraceous and usually
with apical spine. Ray corollas shortly tridentate strap-shaped or absent, disc
corollas quinquelobate tubular. Anther thecae calcarate and caudate. Style
branches with long sweeping hairs consisting of two or three cells separated by
longitudinal walls. Pappus consisting of bi- or triseriate scabrid-barbellate
capillary bristles. x = 9.
[Arctotideae+[Platycarpheae+[Liabeae+[Distephanus+Moquinieae+Vernonieae]]]]
Arctotideae Cass. in
J. Phys. Chim. Hist. Nat. Arts 88: 159. Feb 1819
14/195–210. Heterolepis (4;
H. aliena, H. anomala, H. mitis, H.
peduncularis; Western and Eastern Cape); Arctotheca
(5; A. calendula, A. forbesiana, A. marginata,
A. populifolia, A. prostrata; Mozambique, southern Africa),
Arctotis
(50–60; Angola, southern Africa), Cymbonotus (3; C.
lawsonianus, C. maidenii, C. preissianus; southeastern
South Australia to southeastern Queensland, Tasmania), Dymondia
(1; D. margaretae; Bredasdorp in Western Cape), Haplocarpha
(9–10; Ethiopia, tropical East Africa, South Africa), Berkheya
(75–80; tropical and southern Africa), Cullumia
(15; Northern and Western Cape), Cuspidia (1; C. cernua;
Northern, Western and Eastern Cape), Didelta
(2; D. carnosa, D. spinosa; southern Namibia, Northern and
Western Cape), Gazania
(16–17; tropical and southern Africa, with their highest diversity in the
Cape Provinces), Gorteria
(3; G. corymbosa, G. diffusa, G. personata; southern
Namibia, Northern, Western and Eastern Cape), Heterorhachis (2; H.
aculeata, H. hystrix; Northern and Western Cape), Hirpicium
(12; tropical and southern Africa). – Tropical and southern Africa,
southeastern Australia, Tasmania, with their highest diversity in the Cape
Provinces. Shrubs or herbs. Latex present (Gorteriinae) or absent
(Arctotidinae). Capitula radiate, with imbricate involucral bracts,
outer with foliaceous apex, inner with scarious apex. Ray corollas tri- or
quadrilobate, disc corollas shallowly quinquelobate. Anther thecae calcarate
and caudate or ecaudate. Style with thickened apex and small broadly subulate
or clavate patent sweeping hairs, longest in ring below branching point.
Cypsela sometimes winged. Pappus consisting of uni- or biseriate scales. x = 9.
– The placement of Heterolepis (x = 10) is very unclear. It is
positioned as sister-group either to the Arctotideae or to the
Platycarpheae, or inside the Arctotideae (or even in
Cichorieae), depending on the datasets used.
[Platycarpheae+[Liabeae+[Distephanus+Moquinieae+Vernonieae]]]
Platycarpheae V. A.
Funk et H. Rob., Compositae Newslett. 47: 25. 15 Apr 2009
2/3. Platycarpha (1; P.
glomerata; Eastern Cape to KwaZulu-Natal), Platycarphella (2;
P. carlinoides, P. parvifolia; Namibia, South Africa,
Botswana). – Southern Africa. Perennial herbs. Capitula discoid, with
slightly imbricate involucral bracts. Corollas quinquelobate tubular. Anther
thecae calcarate and ecaudate (or caudate). Style usually hairy distally.
Pappus consisting of scales. x = 9. – According to Funk & Koekemoer
(2011) the distributional pattern of the three species may be explained by
fragmentation of a widespread ancestor due to the rise of the Great Escarpment
combined with changes in the climate.
[Liabeae+[Distephanus+Moquinieae+Vernonieae]]
Liabeae Rydb. in N.
Amer. Fl. 33: 46. 15 Sep 1922
18/c 165. Cacosmia (3; C.
harlingii, C. hieronymi, C. rugosa; Ecuador, Peru),
Ferreyranthus (8; Ecuador, Peru), Oligactis (8; Costa Rica,
Panamá, Colombia, Ecuador, Venezuela), Dillandia (2; D.
chachapoyensis, D. subumbellata; Colombia, Ecuador, Peru),
Sampera (8; Colombia, Ecuador, Peru), Liabum (26; Mexico,
Central America, the West Indies, tropical South America), Sinclairia
(c 30; Mexico, Central America, Colombia), Stephanbeckia (1; S.
plumosa; Bolivia), Microliabum (5; M. candidum, M.
eremophilum, M. humile, M. mulgediifolium, M.
polymnioides; Bolivia, Argentina), Pseudonoseris
(4; P. discolor, P. glandulosa, P. striatum, P.
szyszylowiczii; Peru, Bolivia), Paranephelius (7; P.
asperifolius, P. bullatus, P. ferreyrii, P.
jelskii, P. ovatus, P. uniflorus, P. wurdackii;
Ecuador, Peru, Bolivia, Argentina), Chionopappus (1; C.
benthamii; Peru), Philoglossa (2; P. mimuloides, P.
peruviana; Colombia, Ecuador, Peru, Bolivia), Erato (5; E.
costaricensis, E. polymnioides, E. sodiroi, E.
stenolepis, E. vulcanica; Costa Rica, Panamá, northwestern South
America to Bolivia and Venezuela), ‘Munnozia’ (37; Costa Rica,
Panamá, northwestern South America to northern Argentina and Venezuela;
paraphyletic; incl. Chrysactinium?), Chrysactinium (7; C.
acaule, C. amphothrix, C. breviscapum, C.
caulescens, C. hieracioides, C. rosulatum, C.
wurdackii; Ecuador, Peru; in Munnozia?), Bishopanthus
(1; B. soliceps; Peru)?, Inkaliabum (1; I. diehlii;
Peru). – Mexico, Central America, the West Indies, tropical South America,
with their largest diversity in northwestern South America. Small trees, shrubs
or annual or perennial herbs. Latex usually present. Leaves usually opposite.
Capitula radiate, with graduate involucral bracts. Ray corollas trilobate
strap-shaped, disc corollas quinquelobate, tubular. Anther thecae calcarate and
caudate. Style with sweeping hairs in distal parts. Pappus consisting of long
inner capillary bristles and short outer multiseriate squamellae (sometimes
scales, plumose bristles or absent). x = 9.
[Distephanus+Moquinieae+Vernonieae]
Distephaninae S. C.
Kelley et H. Rob. in V. A. Funk et al., Syst. Evol. Biogeogr. Compositae: 448.
25 Aug 2009
1/>42. Distephanus (>42;
tropical and southern Africa, Socotra, Madagascar, Mauritius, China). –
Trees, shrubs or lianas. Leaves often trinervate or triplinervate. Capitula
discoid, with usually persistent involucral bracts. Anther thecae caudate.
Stylar base thickened. Pappus consisting of biseriate capillary bristles. n =
10. Elemanolides present.
Moquinieae H. Rob. in
Taxon 43: 39. 8 Feb 1994
2/3. Moquinia (2; M.
bojeri: Tanzania; M. racemosa: eastern Brazil),
Pseudostifftia (1; P. kingii; Bahia in Brazil). – Brazil.
Shrubs. Involucral bracts with hyaline margins. Corollas actinomorphic,
quinquelobate. Anther thecae calcarate and caudate. Style swollen and scabrous
near branching point, branches scabrous. Achenes with twin hairs. Pappus
biseriate-capillary. n = ? Guaianolides present.
Vernonieae Cass. in
J. Phys. Chim. Hist. Nat. Arts 88: 203. Mar 1819
134/1.335–1.350.
Acanthodesmos (1; A. distichus; Jamaica), Gorceixia
(1; G. decurrens; southeastern Brazil), Bolanosa (1; B.
coulteri; Mexico), Eremosis (9; Mexico, Central America),
Leiboldia (1; L. serrata; Mexico), Lepidonia (5;
L. callilepis, L. corae, L. lankesteri, L.
mexicana, L. salvinae; Mexico, Central America),
Stramentopappus (1; S. pooleae; Mexico), Albertinia
(1; A. brasiliensis; Brazil), Aynia (1; A.
pseudascaricida; Peru), Chrysolaena (18; tropical South America),
Cololobus (3; C. hatschbachii, C. longiangustatus,
C. rupestris; Brazil), Cyrtocymura (6; C. cincta,
C. harleyi, C. lanuginosa, C. mattos-silvae, C.
saepia, C. scorpioides; southern Mexico, Central America, the
West Indies, tropical South America, with their highest diversity in Brazil),
Dasyanthina (2; D. palustris, D. serrata; eastern
Brazil), Dipterocypsela (1; D. succulenta; Colombia),
Echinocoryne (6; E. echinocephala, E. holosericea,
E. pungens, E. schwenkiaefolia, E. stricta, E.
subulata; Brazil), Eirmocephala (3; E. brachiata, E.
cainarachiensis, E. megaphylla; Central America, the Andes in
Colombia to Bolivia), Gossweilera (2; G. lanceolata, G.
paludosa; Angola), Harleya (1; H. oxylepis; Mexico,
Central America), Heterocypsela (1; H. andersonii; Brazil),
Lessingianthus (c 125; South America, with their highest diversity in
Brazil), Manyonia (1; M. peculiaris; Tanzania),
Mattfeldanthus (2; M. andrade-limae, M. mutisioides;
Brazil), Pseudopiptocarpha (4; P. elaeagnoides, P.
garcia-barrigae, P. schultzii, P. tovarensis; tropical
America), Quechualia (4; Q. cardenasii, Q. fulta,
Q. smithii, Q. trixioides; Bolivia, Argentina),
Sparganophoros (1; S. sparganophora; Central America, the
West Indies, tropical South America), Stenocephalum (6; S.
apiculatum, S. hystrix, S. jucundum, S.
megapotamicum, S. monticola, S. tragiaefolium; tropical
South America), Stilpnopappus (c 20; tropical South America),
Tephrothamnus (2; T. calophyllus, T. paradoxus;
northern tropical South America), Trepadonia (2; T. mexiae,
T. oppositifolia; Peru), Vernonanthura (65–70; Mexico,
Central America, the West Indies, tropical South America), Vernonia
(c 20; tropical to warm-temperate America), Lepidaploa (c 140; Mexico,
Central America, the West Indies, tropical South America), Xiphochaeta
(1; X. aquatica; northeastern South America), Blanchetia (1;
B. heterotricha; northeastern Brazil), Critoniopsis (c 75;
South America, with their largest diversity in the northern Andes),
Cuatrecasanthus (3; C. flexipappus, C. jelskii,
C. sandemanii; Ecuador, Peru), Dasyandantha (1; D.
cuatrecasasiana; Venezuela), Ekmania (1; E. lepidota;
Cuba), Huberopappus (1; H. maigualidae; Venezuela),
Irwinia (1; I. coronata; Brazil), Joseanthus (5;
J. chimborazensis, J. crassilanatus, J.
cuatrecasasii, J. sparrei, J. trichotomus; Colombia,
Ecuador), Piptocarpha (c 50; tropical America), Piptocoma (c
20; Central America, the West Indies, tropical South America), Chresta
(14; central Brazil), Pithecoseris (1; P. pacurinoides;
northern Brazil), Soaresia (1; S. velutina; Brazil),
Centratherum (3; C. australianum, C. cardenasii,
C. punctatum; northern, eastern and southwestern Australia),
Oiospermum (1; O. involucratum; northeastern Brazil),
Anteremanthus (1; A. hatschbachi; Brazil),
Chronopappus (1; C. bifrons; Brazil), Eremanthus (c
30; Brazil, with their highest diversity in semiarid parts of the cerrado),
Lychnophora (35–40; Brazil), Lychnophoriopsis (4; L.
candelabrum, L. damazioi, L. hatschbachii, L.
heterotheca; Brazil), Minasia (6; M. alpestris, M.
cabralensis, M. lewinsohnii, M. pereirae, M.
scapigera, M. splettiae; Brazil), Piptolepis (6; P.
ericoides, P. monticola, P. oleaster, P.
pabstii, P. schultziana; Brazil), Prestelia (1; P.
eriopus; Brazil), Proteopsis (1; P. argentea; southern
Brazil), Bishopalea (1; B. erecta; Brazil),
Heterocoma (1; H. albida; Brazil), Sipolisia (1;
S. lanuginosa; Brazil), Xerxes (1; X. ekmanianum;
Brazil), Caatinganthus (2; C. harleyi, C.
rubropappus; Brazil), Elephantopus
(c 30; tropical and subtropical regions on both hemispheres),
Pseudelephantopus (1; P. spicatus; Colombia),
Rolandra (1; R. fruticosa; tropical America),
Spiracantha (1; S. cornifolia; Central America, Colombia,
Venezuela), Stokesia
(1; S. laevis; southeastern United States), Pacourina (1;
P. edulis; tropical America), Acilepis (32; India to China),
Ageratinastrum (5; A. goetzeanum, A. katangense,
A. lejolyanum, A. palustre, A. polyphyllum; tropical
Africa), Ambassa (1; A. hochstetteri; Ethiopia),
Bechium (2; B. nudicaule, B. rhodolepis;
Madagascar), Bothriocline (c 60; tropical and
southern Africa, Madagascar), Cyanthillium (8; tropical Africa,
Madagascar, tropical Asia), Decastylocarpus (1; D. perrieri;
Madagascar), Dewildemania (8; tropical Africa),
Diaphractanthus (1; D. bomolepis; Madagascar),
Erlangea (16; tropical and southern Africa, Madagascar),
Ethulia (18; tropical Africa, Madagascar, tropical Asia),
Gutenbergia (25–30; tropical Africa), Herderia (1; H.
truncata; tropical West Africa), Hilliardiella (8; eastern and southern Africa, the Arabian Peninsula),
Hoffmannanthus (1; H. abbotianus; tropical Central and East
Africa to Angola, Malawi and Zambia), Hystrichophora (1; H.
macrophylla; Tanzania, extinct?), Iodocephalopsis (1; I.
eberhardtii; northern Thailand)?, Iodocephalus (1; I.
eberhardtii; northern Thailand), Jeffreycia (5; J.
amanuensis, J. hildebrandtii, J. usambarensis, J.
zanzibarensis: Somalia, Kenya, Tanzania; J. zeylanica: Sri
Lanka), Kinghamia (5; K. angustifolia, K.
engleriana, K. foliosa, K. macrocephala, K.
nigritana; tropical West and Central Africa), Koyamasia (1;
K. calcarea; Thailand), Lettowia (1; L. nyassae;
East Africa), Mesanthophora (2; M. brunneri, M.
rojasii; central Paraguay), Msuata (1; M. buettneri;
Congo), Muschleria (1; M. angolensis; Angola), Okia
(2; O. birmanica, O. pseudobirmanica; Burma, Thailand),
Omphalopappus (1; O. newtonii; Angola), Oocephala
(2; O. agrianthoides, O. stenocephala; tropical Africa),
Orbivestus (10–11; tropical and southern Africa),
Phyllocephalum (13; India, Java), Pulicarioidea (1; P.
annamica; Burma, Thailand, Laos, Vietnam), Rastrophyllum (2;
R. apiifolium, R. pinnatipartitum; Tanzania, Zambia),
Telmatophila (1; T. scolymastrum; Brazil),
Vernoniastrum (12; tropical Africa), Brachythrix (7; B.
brevipapposa, B. glomerata, B. lugarensis, B.
malawiensis, B. pawekiae, B. sonchoides, B.
stolzii; Central and East Africa), Cabobanthus (2; C. bullulatus, C. polysphaerus; tropical
Africa), Centauropsis (9; Madagascar), Centrapalus (1; C.
pauciflorus; southern Africa), Nothovernonia (2; N.
amblyolepis, N. purpurea; tropical Africa), Parapolydora
(1; P. fastigiata; Nambia, South Africa, Zimbabwe), Asia),
Aedesia (3; A. engleriana, A. glabra, A.
spectabilis; tropical Africa), Ananthura (1; A.
pteropoda; Central Africa), Baccharoides (c 25; tropical and
southern Africa, India), Camchaya (8; Yunnan, Southeast Asia),
Khasianthus (1; K. subsessilis; India, southern China,
Burma), Lachnorhiza (2; L. micrantha, L.
piloselloides; western Cuba), Linzia (10; southeastern Africa),
Namibithamnus (1; N. obionifolius; Namibia),
Neurolakis (1; N. modesta; Cameroon), Pleurocarpaea
(2; P. denticulata, P. fasciculata; tropical Australia),
Vernonella (12; tropical and southern Africa), Brenandendron
(3; B. donianum, B. frondosum, B. titanophyllum;
tropical Africa), Decaneuropsis (12; India, southern China, Southeast
Asia, West Malesia), Gymnanthemum
(c 40; tropical and southern Africa, Madagascar, tropical Asia to southern
China and Burma), Hesperomannia (3; H. arborescens, H.
arbuscula, H. lydgatei; the Hawaiian Islands), Kurziella
(1; K. gymnoclada; Southeast Asia), Lampropappus (3; L.
eremanthifolia, L. hoffmannii, L. turbinellus; tropical
Africa), Monosis (7; M. aplinii, M. parishii, M.
shevaroyensis, M. talaumifolia, M. travancorica, M.
volkameriifolia, M. wightiana; tropical Asia), Myanmaria
(1; M. calycina; Burma), Oliganthes (6; O. bathiaei,
O. lanuginosa, O. lecomtei, O. pseudocentauropsis,
O. sublanata, O. triflora; Madagascar), Strobocalyx
(9; tropical Asia from India to New Guinea), Tarlmounia (1; T.
elliptica; Thailand), Trichospira (1; T. verticillata;
tropical America), Uniyala (7; southern India, Sri Lanka),
Vinicia (1; V. tomentosa; Minas Gerais in Brazil). –
Tropical and subtropical regions on both hemispheres. Small trees, shrubs,
lianas or herbs. Latex sometimes present. Leaves usually alternate. Involucral
bracts gradate to subequal. Corollas usually actinomorphic, quinquelobate.
Anther thecae calcarate or ecalcarate, caudate or ecaudate. Stylar branches
with long sweeping hairs. Pappus usually capillary. x = 10.
[Corymbioideae+Asteroideae]
Pollen grains fully caveate, without
columellae spanning space above foot layer, and usually with internal foramina;
tectal spines pointed.
Corymbioideae Panero
et V. A. Funk in Proc. Biol. Soc. Washington 115: 910. 30 Dec 2002
1/9. Corymbium
(9; Western and Eastern Cape). – Perennial herbs. C Leaves long, linear,
parallelodromous. Capitulas discoid, with one floret enclosed by two innermost
involucral bracts. Corolla quinquelobate, with linear to oblong and patent
lobes. Pollen grains spheroidal; internal foramina sometimes absent. Ovary
densely hairy. Stylar branches “long”, hairy, with tapering apex. Cypsela
densely pubescent. Pappus consisting of basally connate scales and/or thin
bristles. n = 16; x = 8.
Asteroideae Lindl. in
J. C. Loudon, Encycl. Plant.: 1074. 1829 [‘Astereae’]
1.181/17.360–>17.620.
Cosmopolitan. Laticifers absent. Leaves usually alternate (sometimes opposite).
Female capitula sometimes uniflorous. Trilobate ray florets frequent (female).
Ray corollas tridentate or absent, disc corollas usually actinomorphic. Anthers
sometimes free from each other (especially in anemophilous species), often
without appendage. Anther thecae often ecalcarate and usually ecaudate. Pollen
grains spheroidal, caveate, with cavities separating columellae from foot
layer; colpus ends acute. Tectum double. Stylar hairs often rounded, present
only at stylar apex. Stigmatic areas in two marginal bands. x =
(4–)9–10(–19). ‘4 x 6 bp’ inversion in plastid gene rbcL.
Sesquiterpene lactones at biogenetic levels 3 and 4, benzopyrans, benzofurans,
and 6,8-deoxygenation of flavonoids present. – The probable topology is
[Abrotanella+Doronicum+Senecioneae+[[Calenduleae+[Gnaphalieae+[Astereae+Anthemideae]]]+[Inuleae+[Athroismeae+[Feddeeae+[Helenieae+[Coreopsideae+[[Neurolaeneae+[Tageteae+[Bahieae+Chaenactideae]]]+[[Polymnieae+[Heliantheae+[Millerieae+[Madieae+[Eupatorieae+Perityleae]]]]]]]]]]]]]]]].
Abrotanellinae H.
Rob., G. D. Carr, R. M. King et A. M. Powell in Ann. Missouri Bot. Gard. 84:
896. 1998
1/20. Abrotanella (20;
mountains on New Guinea, eastern Victoria, Tasmania, New Zealand, southernmost
South America). – Perennial herbs (often cushion-plants). Capitula discoid,
with biseriate involucral bracts. Pollen grains “senecioid”. Pappus absent.
x = 9. – The sister-group relationship of Abrotanella is
unresolved.
Doroniceae Panero in
Phytologia 87: 1. 5 Apr 2005
1/c 40. Doronicum
(c 40; Europe except northern parts, the Mediterranean, temperate Asia). –
Perennial herbs. Capitula radiate, with bi- or triseriate equal involucral
bracts. Ray corollas trilobate strap-shaped, disc corollas quinquedentate
tubular. Pappus usually consisting of uni- or biseriate barbellate bristles
(sometimes absent). x = 30. – The sister-group relationship of Doronicum
is unresolved.
Senecioneae Cass. in
J. Phys. Chim. Hist. Nat. Arts 88: 195. Mar 1819
140/3.200–3.235.
Blennosperma (4; B. bakeri, B. nanum: California;
B. cretacea: southern Venezuela, northwestern Brazil; B.
chilense: Chile), Crocidium (2; C. multicaule, C.
pugetense; southwestern Canada, western United States), Ischnea
(5; I. brassii, I. capellana, I. elachoglossa,
I. keysseri, I. korythoglossa; New Guinea),
Chersodoma (9; the Andes), Homogyne
(2; H. alpina, H. discolor; mountains in Central Europe),
Endocellion (1; E. glaciale; Siberia), Petasites
(c 20; temperate, arctic and alpine regions on the Northern Hemisphere), Tussilago
(1; T. farfara; Europe, the Mediterranean, North Africa, temperate
Asia), Farfugium (2; F. hiberniflorum, F. japonicum;
East Asia), Miricacalia (1; M. makinoana; Japan),
Dendrocacalia (1; D. crepidifolia; Bonin Islands),
’Parasenecio’ (c 70; Russia, East Asia, Alaska; polyphyletic), Sinacalia
(4; S. caroli, S. davidii, S. macrocephala, S.
tangutica; China; in Parasenecio?), Dicercoclados (1;
D. triplinervis; China), Syneilesis (6; S.
aconitifolia, S. australis, S. intermedia, S.
palmata, S. subglabrata, S. tagawae; East Asia), ’Ligularia’
(150–155; eastern Europe, temperate Asia, with their
highest diversity in China; paraphyletic), Ligulariopsis (1; L.
shichuana; China), ’Cremanthodium’
(c 75; the Himalayas, Tibet, western and southern China; polyphyletic),
Nemosenecio (6; N. concinnus, N. formosanus, N.
incisifolius, N. nikoensis, N. solenoides, N.
yunnanensis; China, Japan, Taiwan), Sinosenecio (c 45; China,
Southeast Asia, northwestern North America), Hainanecio (1; H.
hainanensis; Hainan), Tephroseris
(c 40; temperate and arctic regions on the Northern Hemisphere),
Psacaliopsis (4; P. macdonaldii, P. paneroi, P.
pinetorum, P. purpusii; Mexico, Guatemala), Psacalium
(45–50; southwestern United States, Mexico, Central America),
Pippenalia (1; P. delphiniifolia; Mexico),
Digitacalia (5; D. chiapensis, D. crypta, D.
hintoniorum, D. jatrophoides, D. napeifolia; Mexico),
Rugelia (1; R. nudicaulis; southeastern United States),
Arnoglossum (8; eastern United States), Robinsonecio (2;
R. gerberifolius, R. porphyresthes; Mexico, Guatemala), Roldana
(c 55; southern United States, Mexico, Central America), Nelsonianthus
(1; N. tapianus; Mexico, Guatemala), Telanthophora (5; T.
arborescens, T. bartlettii, T. grandifolia, T.
serraquitchensis; southern Mexico, Central America), Villasenoria
(1; V. orcuttii; Mexico), Pittocaulon (2; P.
hintonii, P. velatum; Mexico, Central America),
Barkleyanthus (1; B. salicifolius; southwestern United
States, Mexico, Central America), Yermo (1; Y.
xanthocephalus; Wyoming), Rainiera (1; R. stricta;
northwestern United States), Cacaliopsis (1; C. nardosmia;
western United States), Luina (2; L. hypoleuca, L.
serpentina; northwestern North America), Tetradymia (10; North
America), Lepidospartum (3; L. burgessii, L.
latisquamum, L. squamatum; southwestern United States),
Paracalia (3; P. jungioides, P. lopezii, P.
pentamera; Peru, Bolivia), Paragynoxys (11; northwestern tropical
South America), Gynoxys (125–130; Central America, northern South
America to Peru), Nordenstamia (c 15; western Ecuador, western Peru to
Argentina), Aequatorium (23; Peru, Bolivia, northern Argentina),
Acrisione (1; A. denticulata; central Chile), Brachyglottis
(c 40; New Zealand, Three Kings Islands, Chatham Islands, one species, B.
brunonis, in southeastern New South Wales and Tasmania; paraphyletic?),
Capelio (3; C. caledonica, C. tabularis, C.
tomentosa; Western Cape), Papuacalia (17; mountains on New
Guinea), Brachionostylum (1; B. pullei; New Guinea),
Pladaroxylon (1; P. leucadendron; St. Helena),
Lachanodes (1; L. arborea; St. Helena), Scrobicaria
(3; S. aquifolia, S. ilicifolia, S. soatana;
northeastern South America), Shafera (1; S. platyphylla;
eastern Cuba), Mattfeldia (1; M. triplinervia; Hispaniola),
Ekmaniopappus (1; E. mikanioides; Hispaniola),
Herodotia (1; H. haitiensis; Hispaniola), Leonis (1;
L. trineura; Cuba, Hispaniola), Nesampelos (3; N.
alainii, N. hotteana, N. lucens; Hispaniola),
Zemisia (1; Z. discolor; Jamaica), Antillanthus (17;
Cuba), Elekmania (9; Hispaniola), Herreranthus (1; H.
rivalis; Cuba), Oldfeltia (1; O. polyphlebia; Cuba),
Odontocline (6; O. dolichantha, O. fadyenii, O.
glabra, O. hollickii, O. laciniata, O.
tercentenariae; Jamaica), Lundinia (1; L. plumbea; Cuba,
Hispaniola), Jacmaia (4–5; J. cooperi, J. incana,
J. megaphylla, J. multivenia; Costa Rica, Jamaica),
Ignurbia (1; I. constanzae; Hispaniola), Jessea (4;
J. cooperi, J. gunillae, J. megaphylla, J.
multivenia; Costa Rica, Panamá), Lordhowea (1; L.
insularis; Lord Howe Island), Arrhenechthites (7; A.
alba, A. haplogyna, A. hydrangeoides, A.
mastigothrix, A. mixta, A. novoguineensis, A.
tomentella; mountains on Sulawesi, New Guinea and in eastern New South
Wales and Victoria), Bethencourtia
(1–3; B. hermosae, B. palmensis, B. rupicola; the
Canary Islands), Pericallis
(15; Macaronesia), Cineraria
(c 50; tropical and southern Africa, Madagascar, southwestern Arabian
Peninsula), Bertilia (1; B. hantamensis; southwestern
Northern Cape), Bolandia (5; B. argillacea, B.
elongata, B. glabrifolia, B. pedunculosa, B.
pinnatifida; Western and Eastern Cape, Drakensberg in Lesotho),
Mesogramma (1; M. apiifolium; Gariep area in South Africa),
Stilpnogyne (1; S. bellidioides; Western Cape),
Packera (c 65; Siberia, North America), Dorobaea (3; D.
callacallensis, D. laciniata, D. pimpinellifolia; the
Andes), 'Senecio'
(1.500 – 1.600?; cosmopolitan except polar regions; paraphyletic),
Lomanthus (20; Ecuador, Peru, Bolivia, Argentina), Jacobaea
(40–45; Europe, temperate Asia), Solanecio (16; tropical and
southern Africa, Madagascar, Yemen), Kleinia
(c 60; the Canary Islands, Africa, Madagascar, the Arabian Peninsula to
southern India and Sri Lanka), Gynura (c 45; tropical regions in the
Old World), Erechtites (9; Australia, New Zealand, North to South
America), Io (1; I. amboadrombensis; Madagascar),
Crassocephalum (23; tropical and subtropical Africa, Madagascar, the
Mascarene Islands, Yemen), Hoehnephytum (3; H. almasense,
H. imbricatum, H. trixoides; Brazil), Pentacalia
(220–230; Central and South America), Monticalia (11–13?; Central
and South America, with their largest diversity in the Andes),
Dendrophorbium (c 55; South America), Graphistylis (9;
southern Brazil), Caxamarca (1; C. sanchezii; northern Peru),
Pseudogynoxys (18; tropical South America), Arbelaezaster (1;
A. ellsworthii; Colombia), Dresslerothamnus (3; D.
angustiradiatus, D. gentryi, D. peperomioides; Central
America, Colombia), Cabreriella (2; C. oppositicordia, C.
sanctae-martae; Colombia), Charadranaetes (1; C.
durandii; Costa Rica), Misbrookea (1; M. strigosissima;
Peru, Bolivia), Xenophyllum (21; the Andes), Werneria (19;
the Andes), Oresbia (1; O. heterocarpa; Western Cape),
Lamprocephalus (1; L. montanus; Western Cape),
Phaneroglossa (1; P. bolusii; Northern and Western Cape),
Dendrosenecio (11; mountains in Central and tropical East Africa), Euryops
(c 100; Africa, the Arabian Peninsula, Socotra), ’Othonna’
(c 140; tropical and southern Africa, Tasmania, with their highest diversity in
southern Africa; diphyletic), Caputia (5; C. medley-woodii,
C. oribiensis, C. pyramidata, C. scaposa, C.
tomentosa; eastern South Africa), Crassothonna (13; southwestern
South Africa), Gymnodiscus
(2; G. capillaris, G. linearifolius; Northern and Western
Cape), Hertia (9; northern and southern Africa, southwestern Asia),
Lopholaena (16; tropical and southern Africa), Cadiscus (1;
C. aquaticus; Western Cape; in Senecio?), Stenops
(2; S. helodes, S. zairensis; tropical Africa),
Oligothrix (1; O. gracilis; Cedarberg Mountains in Western
Cape), Steirodiscus
(6; S. capillaceus, S. gamolepis, S. linearilobus,
S. schlechteri, S. speciosus, S. tagetes; Northern
and Western Cape), Bafutia (1; B. tenuicaulis; Cameroon),
Psednotrichia (2; P. newtonii, P. xyridopsis;
Angola), Emilia
(c 115; tropical and southern Africa, tropical Asia), Angeldiazia (1;
A. weigendii; northern Peru), Emiliella (6; E.
biensis, E. drummondii, E. epapposa, E. exigua,
E. palhinhana, E. zambiensis; Angola, Zambia),
Faujasiopsis (3; F. boivinii, F. flexuosa, F.
reticulata; the Mascarene Islands), Faujasia (4; F.
cadetiana, F. pinifolia, F. salicifolia, F.
squamosa; Réunion), Eriotrix (2; E. commersonii, E.
lycopodioides; Réunion), Hubertia (22; Madagascar, the Mascarene
Islands), Humbertacalia (8; H. amplexifolia, H.
coursii, H. leucopappa, H. neoalleizettii, H.
pyrifolia, H. racemosa, H. volute: Madagascar; H.
tomentosa: Réunion), Parafaujasia (2; P. fontinalis,
P. mauritiana; the Mascarene Islands), Synotis (c 50; the
Himalayas, Tibet, China), Mikaniopsis (c 15; tropical and southern
Africa), Delairea
(1; D. odorata; Western and Eastern Cape, KwaZulu-Natal, Lesotho),
Cissampelopsis (11; tropical Asia), Austrosynotis (1; A.
rectirama; southern tropical Africa), Dauresia (1; D.
alliariifolia; Namibia), Adenostyles
(9; Central, southern and easern Europe, Corsica, the Caucasus),
Caucasalia (4; C. macrophylla, C. parviflora, C.
pontica, C. similiflora; Turkey, the Caucasus),
Dolichorrhiza (4; D. caucasica, D. correvoniana,
D. persica, D. renifolia; the Caucasus, Iran),
Iranecio (16; southeastern Europe to Iran). – Cosmopolitan except
polar regions. Trees, shrubs, lianas, herbs (including epiphytes). Capitula
radiate, disciform or discoid, with usually uniseriate (sometimes bi- or
multiseriate) equal involucral bracts. Ray corollas often tridentate giving
impression of being split zygomorphic (modification of [2:3]-bilabiate corolla,
with reduced adaxial lobes), disc corollas quadri- or quinquelobate campanulate
or tubular. Anther thecae ecalcarate and caudate or ecaudate. Pollen grains
caveate, usually with solid columellae. Style simple or bilobate, with sweeping
hairs in apical tuft or on stylar branches. Pappus usually consisting of
barbellate bristles (sometimes absent). x = 10. Diester pyrrolizidine
alkaloids, eremophilane types sesquiterpene lactones. Polyacetylenes absent.
[[Calenduleae+[Gnaphalieae+[Astereae+Anthemideae]]]+[Inuleae+[Athroismeae+[Feddeeae+[Helenieae+[Coreopsideae+[[Neurolaeneae+[Tageteae+[Bahieae+Chaenactideae]]]+[[Polymnieae+[Heliantheae+[Millerieae+[Madieae+[Eupatorieae+Perityleae]]]]]]]]]]]]]]]
[Calenduleae+[Gnaphalieae+[Astereae+Anthemideae]]]
Calenduleae Cass. in
J. Phys. Chim. Hist. Nat. Arts 88: 161. Feb 1819
4/c 119. Calendula
(12; Central and South Europe, Macaronesia, the Mediterranean to Iran), Osteospermum
(c 80; tropical eastern and southern Africa, northeastern Africa, Somalia, the
Arabian Peninsula, Jordan, one species, O. sanctae-helenae, on St.
Helena), Dimorphotheca
(19; tropical and southern Africa), Garuleum (8; southern Africa). –
Europe, Macaronesia, the Mediterranean, Africa, St. Helena, southwestern Asia,
with their largest diversity in the Cape Provinces. Small trees, shrubs or
herbs. Leaves alternate or opposite. Capitula radiate, with uni- to triseriate
involucral bracts, with or without hyaline margins. Disc corollas
quinquelobate. Anther thecae ecalcarate and caudate. Exine without columellae.
Style simple or bilobate, with sweeping hairs distally. Cypsela terete or
flattened, sometimes curved, rostrate, winged or fenestrate. Exocarp sometimes
fleshy. Pappus absent. x = 10.
[Gnaphalieae+[Astereae+Anthemideae]]
Gnaphalieae (Cass.)
Lecoq et Juill., Dict. Rais. Term. Bot.: 296. 1831
175/1.995–2.020.
Pseudognaphalium (80–90; temperate and subtropical regions on both
hemispheres), Achyrocline (c 45; tropical Africa, Madagascar, South
America), Ammobium (3; A. alatum, A. craspedioides,
A. plantagineum; southeastern Queensland, eastern New South Wales,
eastern Victoria, Tasmania), Asteridea (10; southwestern Western
Australia, southern South Australia, western Victoria), Pterochaeta
(1; P. paniculata; southwestern Western Australia), Podolepis
(20; Australia, Tasmania), ‘Chrysocephalum’ (9; Australia,
Tasmania; polyphyletic), ‘Helichrysum’ (>500?; Europe,
Macaronesia, the Mediterranean, Africa, Madagascar, Asia, Australia, with their
highest diversity in the Cape Provinces; non-monophyletic), Waitzia
(5; W. acuminata, W. corymbosa, W. nitida, W.
podolepis, W. suaveolens; southern Australia),
‘Anemocarpa’ (3; A. calcicola, A. podolepidium,
A. saxatilis; central and southeastern Australia; non-monophyletic),
Rutidosis (9; Australia), Leptorhynchos (c 10; southern and
southeastern Australia, Tasmania), Triptilodiscus (1; T.
pygmaeus; southern Australia, Tasmania), Acomis (4; A.
acoma, A. bella, A. kakadu, A. macra; northern
Northern Australia, Queensland, northeastern New South Wales),
Leucochrysum (5; L. albicans, L. fitzgibbonii,
L. graminifolium, L. molle, L. stipitatum;
Australia, Tasmania), ‘Coronidium’ (9; eastern Australia,
Tasmania; non-monophyletic), Xerochrysum (8; Australia, Tasmania),
Anaphalis (110–115; temperate and alpine Asia and North America);
Libinhania (13; Socotra), Omalotheca (5; O.
afghanica, O. norvegica, O. stewartii, O.
supina, O. sylvatica; arctic and temperate regions in Eurasia and
northern North America), Plecostachys (2; P. polifolia,
P. serpyllifolia; Western and Eastern Cape, KwaZulu-Natal, Swaziland),
Gnaphalium (c 40; cosmopolitan), Leontopodium (c 60;
mountains in Central Europe to China and Burma), Ifloga (16; the
Canary Islands, the Mediterranean, northern to southern Africa, southwestern
Asia), Filago (12; temperate and subtropical regions in the Old
World), Logfia (8–12; L. aberrans, L. arizonica,
L. arvensis, L. depressa, L. filaginoides, L.
gallica, L. minima, L. paradoxa; Europe, the
Mediterranean to Afghanistan, North America), Antennaria (c 60;
temperate, arctic and alpine regions on both hemispheres), Chevreulia
(7; C. acuminata, C. diemii, C. gnaphalioides,
C. lycopodioides, C. pusilla, C. sarmentosa, C.
xeranthemoides; South America, Falkland Islands, Tristan da Cunha),
Cuatrecasasiella (2; C. argentina, C. isernii; the
Andes from Ecuador to northern Argentina), Jalcophila (4; J.
boliviensis, J. colombiana, J. ecuadorensis, J.
peruviana; the Andes in Colombia, Ecuador, Peru and Bolivia),
Loricaria (22; the Andes from Colombia to Bolivia),
Luciliocline (13; the Andes in Venezuela to Peru, Bolivia, Chile and
Argentina), Mniodes (4; M. andina, M. aretioides,
M. coarctata, M. pulvinata; the Andes in Peru, Bolivia and
northern Chile), Novenia (1; N. acaulis; Peru, northwestern
Argentina), ‘Gamochaeta’ (c 60; North America to southern South
America, one species, G. alpina, in subantarctic southern Andes;
polyphyletic), Belloa (1; B. chilensis; the Andes in southern
Chile and southern Argentina), Chionolaena (27; central Mexico,
Central America, northern Colombia, southern Venezuela, southern Brazil),
Berroa (1; B. gnaphalioides; subtropical South America),
Facelis (4; F. brachyantha, F. lasiocarpa, F.
plumosa, F. retusa; South America north to Ecuador, Bolivia,
Brazil and Uruguay), Micropsis (5; M. australis, M.
dasycarpa, M. nana, M. ostenii, M. spathulata;
southern Brazil, Paraguay, Uruguay, northeastern Argentina, central Chile),
Lucilia (9; South America). Unplaced Gnaphalieae:
Acanthocladium (1; A. dockeri; eastern South Australia,
western New South Wales), Actinobole (4; A. condensatum,
A. drummondianum, A. oldfieldianum, A. uliginosum;
southern and central Australia), Alatoseta (1; A. tenuis;
Northern and Western Cape), Amphiglossa (11–12; southern Africa),
Anaphaloides (8; New Guinea, New Zealand), Anaxeton (10;
Western Cape), Ancistrocarphus (2; A. filagineus, A.
keilii; California), Anderbergia (6; A. elsiae, A.
epaleata, A. fallax, A. rooibergensis, A.
ustulata, A. vlokii; Western Cape), Angianthus (19;
western, central and southern Australia, Tasmania), Antithrixia (1;
A. flavicoma; Namaqualand in Western Cape), Apalochlamys (1;
A. spectabilis; southeastern South Australia, Victoria, Tasmania),
Argentipallium (6; A. blandowskianum, A. dealbatum,
A. niveum, A. obtusifolium, A. spiceri, A.
tephrodes; southwestern Western Australia, southeastern South Australia,
Victoria, Tasmania), Argyroglottis (1; A. turbinata;
southwestern Western Australia), Argyrotegium (4; A.
fordianum, A. mackayi, A. nitidulum, A.
poliochlorum; eastern New South Wales, Victoria, Tasmania, New Zealand),
Arrowsmithia (1; A. styphelioides; Eastern Cape),
Athrixia (15; tropical and southern Africa, Madagascar),
Atrichantha (1; A. gemmifera; Western Cape),
Basedowia (1; B. tenerrima; northern South Australia),
Bellida (1; B. graminea; southwesternmost Western Australia),
Blennospora (3; B. doliiformis, B. drummondii,
B. phlegmatocarpa; southwestern Western Australia, southeastern South
Australia, western Victoria), Bryomorphe (1; B. aretioides;
Western Cape), Callilepis (5; C. caerulea, C.
lancifolia, C. laureola, C. leptophylla, C.
salicifolia; South Africa, Swaziland), Calocephalus (9;
Australia, Tasmania), Calomeria (2; C. africana: Mozambique;
C. amaranthoides; eastern New South Wales, Victoria),
Calotesta (1; C. alba; Western Cape), Cassinia
(35–40; eastern and southeastern Australia, Tasmania), Castroviejoa
(2; C. frigida, C. montelinasana; Corsica, Sardinia),
Catatia (2; C. attenuata, C. cordata; Madagascar),
Cephalipterum (1; C. drummondii; southwestern Western
Australia, western South Australia), Cephalosorus (1; C.
carpesioides; westernmost Western Australia), Chamaepus (1;
C. afghanicus; Afghanistan), Chiliocephalum (2; C.
schimperi, C. tegetum; Ethiopia), Chondropyxis (1;
C. halophila; southwestern Western Australia, southern South
Australia), Chthonocephalus (7; C. muellerianus, C.
multiceps, C. oldfieldianus, C. pseudevax, C.
spathulatus, C. tomentellus, C. viscosus; southern
Australia), Cladochaeta (1; C. candidissima; the Caucasus),
Comborhiza (2; C. longipes, C. virgata; Western
Cape, KwaZulu-Natal), Craspedia (c 25; southwestern Western Australia,
southeastern Australia, Tasmania, New Zealand), Cremnothamnus (1;
C. thomsonii; southern Northern Territory, northern South Australia),
Cymbolaena (1; C. griffithii; southwestern to Central Asia),
Decazesia (1; D. hecatocephala; westernmost Western
Australia), Denekia (1; D. capensis; southern and
southeastern Africa), Dielitzia (1; D. tysonii; western
Western Australia), Disparago (9; Western and Eastern Cape,
KwaZulu-Natal), Dithyrostegia (2; D. amplexicaulis, D.
gracilis; southwestern Western Australia), Dolichothrix (1;
D. ericoides; Western and Eastern Cape), Edmondia (3; E.
fasciculata, E. pinifolia, E. sesamoides; Western Cape),
Elytropappus (10; Northern, Western and Eastern Cape),
Epitriche (1; E. demissus; southwesternmost Western
Australia), Eriochlamys (4; E. behrii, E. cupularis,
E. eremaea, E. squamata; southeastern and central Australia),
Erymophyllum (5; E. compactum, E. glossanthus,
E. hemisphaericum, E. ramosum, E. tenellum;
southwestern Western Australia, western South Australia), Euchiton
(17; East Asia, New Guinea, Australia except northwestern parts, Tasmania, New
Zealand), Evacidium (1; E. discolor; Sicily, North Africa),
Evax (1–2; E. arenaria, E. multicaulis; the
Mediterranean to Central Asia), Ewartia (5; E. catipes,
E. meredithae, E. nubigena, E. planchonii, E.
sinclairii; southeasternmost New South Wales, eastern Victoria, Tasmania),
Ewartiothamnus (1; E. sinclairii; New Zealand),
Feldstonia (1; F. nitens; western Western Australia),
Fitzwillia (1; F. axilliflora; southwestern Western
Australia), Galeomma (2; G. oculus-cati, G.
stenolepis; Namibia, Northern Cape, Botswana), Gilberta (1;
G. tenuifolia; southwestern Western Australia), Gilruthia (1;
G. osbornii; western Western Australia), Gnaphaliothamnus (1;
G. lavandulaceum; Mexico, Guatemala), Gnephosis (17;
Australia), Gnomophalium (1; G. pulvinatum; the
Mediterranean), Gratwickia (1; G. monochaeta; South
Australia), Haeckeria (3; H. cassiniiformis, H.
punctata, H. punctulata; southeastern South Australia),
Haegiela (1; H. tatei; southwestern Western Australia,
southeastern South Australia, western Victoria), Haptotrichion (2;
H. colwillii, H. conicum; Carnarvon District in westernmost
Western Australia), Helichrysopsis (1; H. septentrionalis;
coastal Mozambique, KwaZulu-Natal), Hesperevax (3; H.
acaulis, H. caulescens, H. sparsiflora; western United
States), Humeocline (1; H. madagascariensis; Madagascar),
Hyalochlamys (1; H. globifera; southwestern Western
Australia), Hyalosperma (9; southwestern Western Australia,
southeastern Australia, Tasmania), Hydroidea (1; H. elsiae;
Western Cape), Ixiolaena (1; I. viscosa; southern Australia),
Ixodia (1; I. flindersica; southeastern South Australia,
Victoria), Lachnospermum (4; L. fasciculatum, L.
imbricatum, L. neglectum, L. umbellatum; Western Cape),
Langebergia (1; L. canescens; Western Cape),
Lasiopogon (9; Spain, Africa to India), Lawrencella (2;
L. davenportii, L. rosea; southwestern and central
Australia), Leiocarpa (10; Madagascar, Australia), Lemooria
(1; L. burkittii; Australia)?, Lepidostephium (2; L.
asteroides, L. denticulatum; Eastern Cape, KwaZulu-Natal),
Leucogenes (4; L. grandiceps, L. leontopodium,
L. neglecta, L. tarahaoa; New Zealand incl. Stewart Island),
Leucophyta (1; L. brownii; coastal areas in southwestern
Western Australia to eastern Victoria and Tasmania), Leysera (3;
L. gnaphalodes, L. leyseroides, L. tenella; southern
and northern Africa, the Arabian Peninsula to Pakistan), Macowania
(12; Ethiopia, southern Africa, Yemen), Metalasia (52; South Africa,
Lesotho, with their highest diversity in Western Cape), Mexerion (2;
M. mexicanum, M. sarmentosum; Mexico), Micropus (4;
M. supinus: the Iberian Peninsula; M. dasycarpus: the Middle
East to Iran; M. amphibolus, M. californicus: southwestern
United States, northern Baja California), Millotia (16; southern
Australia, Tasmania), Myriocephalus (c 15; Australia),
Neotysonia (1; N. phyllostegia; western Western Australia),
Nestlera (1; N. biennis; Northern and Western Cape),
Odixia (2; O. achlaena, O. angusta; Tasmania),
Oedera (18; Western and Eastern Cape), Oreoleysera (1; O.
montana; Western Cape), Ozothamnus (c 55; Australia except
northwestern parts, Tasmania, New Caledonia, New Zealand),
Parantennaria (1; P. uniceps; eastern Victoria, southeastern
New South Wales), Pentatrichia (5–6; P. alata, P.
avasmontana, P. integra, P. kuntzei, P.
petrosa, P. rehmii; Namibia, Northern and Western Cape,
Mpumalanga), Petalacte (1; P. coronata; Western Cape),
Phaenocoma (1; P. prolifera; Western Cape),
Phagnalon (28; the Canary Islands, the Mediterranean, southwestern
Asia), Pithocarpa (2; P. corymbulosa, P. pulchella;
southwestern Western Australia), Podotheca (6; P. chrysantha,
P. fuscescens, P. gnaphalioides, P. pritzelii,
P. uniseta, P. wilsonii; southwestern Western Australia,
southern South Australia, Victoria, southwesternmost New South Wales),
Pogonolepis (2; P. muelleriana, P. stricta; southwestern and southern
Australia), Polycalymma (1; P. stuartii; southern and central
Australia), Psilocarphus (6; P. brevissimus, P.
chilensis, P. elatior, P. globiferus, P.
oregonus, P. tenellus; tropical regions on both hemispheres,
north to western United States, south to southern South America),
Pterygopappus (1; P. lawrencei; Tasmania), Quinetia
(1; Q. urvillei; southwestern Western Australia, southeastern South
Australia, western Victoria), Quinqueremulus (1; Q. linearis;
western Western Australia), Rachelia (1; R. glaria; South
Island in New Zealand), Raoulia (26; New Guinea, New Zealand),
Raouliopsis (2; R. pachymorpha, R. seifrizii; the
Andes in Colombia), Relhania (13; Northern, Western and Eastern Cape,
KwaZulu-Natal, Free State, Lesotho), Rhodanthe (46; Australia,
Tasmania), Rhynchopsidium (2; R. pumilum, R.
sessiliflorum; Northern and Western Cape), Rosenia (4; R.
glandulosa, R. humilis, R. oppositifolia, R.
spinescens; Namibia, Northern, Western and Eastern Cape, North-West, Free
State, Botswana), Schoenia (5; S. ayersii, S.
cassiniana, S. filifolia, S. macivorii, S.
ramosissima; Australia except northern parts), Siloxerus (4;
S. filifolius, S. humifusus, S. multiflorus, S.
pygmaeus; southwestern Western Australia, southeastern South Australia,
Victoria, southeastern New South Wales, Tasmania), Sondottia (2;
S. connata, S. glabrata; southwestern Western Australia),
Stenocline (2; S. chionaea, S. ericoides;
Madagascar, Mauritius), Stenophalium (4; S. almasense, S.
chionaeum, S. eriodes, S. heringeri; Brazil),
Stoebe (34; tropical and southern Africa, Madagascar, the Mascarene
Islands), Stuartina (2; S. hamata, S. muelleri;
southeastern Australia), Stylocline (7; S. citroleum, S.
gnaphaloides, S. intertexta, S. masonii, S.
micropoides, S. psilocarphoides, S. sonorensis;
southwestern United States, northwestern Mexico), ‘Syncarpha’ (c
30; Western and Eastern Cape; non-monophyletic), Syncephalum (1;
S. arbutifolium; Madagascar), Taplinia (1; T.
saxatilis; central Western Australia), Tenrhynea (1; T.
phylicifolia; Eastern Cape, KwaZulu-Natal, Northern Province, Mpumalanga,
Swaziland), Thiseltonia (1; T. gracillima; southern central
Western Australia), Tietkensia (1; T. corrickiae; western
central Australia), Trichanthodium (4; T. baracchianum,
T. exilis, T. scarlettianum, T. skirrophorum;
southern Australia), Troglophyton (6; T. acocksianum, T.
capillaceum, T. elsiae, T. leptomerum, T.
parvulum, T. tenellum; Namibia, Northern, Western and Eastern
Cape, KwaZulu-Natal, Free State), Vellereophyton (7; V.
dealbatum, V. felinum, V. gracillimum, V.
lasianthum, V. niveum, V. pulvinatum, V.
vellereum; Northern, Western and Eastern Cape). – Subcosmopolitan, with
their highest diversity in southern Africa and Australia. Shrubs or herbs.
Capitula usually discoid or disciform, with imbricate multiseriate involucral
bracts, usually with upper part papery and basal part cartilaginous. Outer
corollas usually filiform or absent. Anther thecae ecalcarate and caudate.
Ectexine “gnaphalioid”, with outer columellate layer and basal irregularly
interlaced layer. Pappus usually consisting of plumose or barbellate to scabrid
capillary bristles. x = 7.
[Astereae+Anthemideae]
Astereae Cass. in J.
Phys. Chim. Hist. Nat. Arts 88: 195. Mar 1819
218/3.400–3.440. Commidendrum
(5; C. burchellii, C. robustum, C. rotundifolium,
C. rugosum, C. spurium; St. Helena), Cuniculotinus
(1; C. gramineus; western United States), Doellingeria
(10; temperate Asia, North America), Ericameria
(c 35; southwestern Canada, western United States, northwestern Mexico),
Eucephalus (10; southwestern Canada, western United States), Eurybia
(22; Europe, temperate Asia, Canada, United States), Heteroplexis (2;
H. impressinervia, H. incana; China), Ionactis (5;
I. alpina, I. caelestis, I. elegans, I.
linariifolia, I. stenomeres; southern Canada, United States),
Melanodendron (1; M. integrifolium; St. Helena), Microglossa
(8; tropical and southern Africa, Madagascar, the Mascarene Islands, tropical
Asia), Nannoglottis
(4; N. carpesioides, N. hookeri, N. latisquama,
N. yunnanensis; western China), Oclemena
(3; O. acuminata, O. nemoralis, O. reticulata;
southern Canada, United States), Oreostemma (3; O. alpigenum,
O. elatum, O. peirsonii; western United States), Psiadia
(40–45; tropical Africa, Madagascar, tropical Asia), Sarcanthemum (1; S.
coronopus; Rodrigues), Tonestus (4; T. eximius, T.
graniticus, T. lyallii, T. pygmaeus; southwestern
Canada, western United States), Vernoniopsis (2; V. caudata,
V. lokohensis; Madagascar), Amellus (12; southern Africa),
Chamaegeron (4; C. asterellus, C. bungei, C.
keredjensis, C. oligocephalus; Central Asia), Chrysocoma
(c 25; southern Africa, with their highest diversity in Western Cape),
Engleria (2; E. africana, E. decumbens; Angola,
southern Africa), Felicia
(c 85; tropical and southern Africa, Ethiopia, the Arabian Peninsula),
Gymnostephium (8; G. angustifolium, G. ciliare,
G. corymbosum, G. fruticosum, G. gracile, G.
hirsutum, G. laeve, G. papposum; Western Cape),
Jeffreya (2; J. palustris, J. petitiana; tropical
Africa), Lachnophyllum (2; L. gossypinum, L.
noeanum; western to Central Asia), Nolletia (9; southern Spain,
North and southern Africa), Poecilolepis (2; P. ficoidea,
P. maritima; Western and Eastern Cape), Polyarrhena
(4; P. imbricata, P. prostrata, P. reflexa, P.
stricta; Western Cape), Roodebergia (1; R. kitamurana;
Western Cape), Zyrphelis (9; tropical and southern Africa),
Achnophora (1; A. tatei; southeastern South Australia),
Aylacophora (1; A. deserticola; Nequén in Patagonian
Argentina), Aztecaster (2; A. matudae, A.
pyramidatus; Mexico), Blakiella (1; B. bartsiifolia;
Colombia, Venezuela), Cabreraea (1; C. andina; La Rioja and
Catamarca in Argentina), Celmisia
(c 70; southeastern New South Wales, Victoria, Tasmania, New Zealand),
Chiliophyllum (3; C. andinum, C. densifolium, C.
fuegianum; Mendoza in Argentina), Chiliotrichiopsis (4; C.
keidelii, C. ledifolia, C. mendocina, C.
peruviana; southern Peru, Bolivia, northern Argentina),
Chiliotrichum (4; C. angustifolium, C. diffusum,
C. rosmarinifolium, C. tenue; Chile, Argentina and southern
Bolivia to Tierra del Fuego), Damnamenia (1; D. vernicosa;
Auckland Islands, Campbell Islands), Diplostephium (c 110; the Andes
from Colombia to northern Chile), Floscaldasia (2; F.
azorelloides, F. hypsophila; the Andes in Colombia and Ecuador),
Flosmutisia (1; F. paramicola; Colombia), Guynesomia
(1; G. scoparia; central Chile), Haroldia (1; H.
mendocina; Mendoza in Argentina), Hinterhubera (8; the Andes),
Katinasia (1; K. cabrerae; Mendoza and Nequén in Argentina),
Laestadia (6; L. costaricensis, L. domingensis,
L. lechleri, L. muscicola, L. pinifolia, L.
rupestris; the West Indies, tropical Andes), Lepidophyllum (1;
L. cupressiforme; Chile, Argentina to Tierra del Fuego),
Llerasia (11; Colombia to southern Bolivia), Madagaster (5;
M. andohahelensis, M. madagascariensis, M.
mandrarensis, M. saboureaui, M. senecionoides;
Madagascar), Mairia
(6; M. burchellii, M. coriacea, M. crenata, M.
hirsuta, M. petiolata, M. robusta; Western Cape),
Nardophyllum (7; N. armatum, N. bryoides, N.
cabrerae, N. chiliotrichoides, N. genistoides, N.
lanatum, N. obtusifolium; southern Andes in Chile and Argentina),
Novenia (1; N. acaulis; the Andes), Ocyroe (1;
O. armata; San Juan to Jujuy in Argentina, northern Chile, southern
Bolivia), Olearia
(c 180; New Guinea, Australia, Tasmania, New Zealand), Oritrophium
(18; Mexico, the Andes), Pachystegia
(3; P. insignis, P. minor, P. rufa; South Island of
New Zealand), Pacifigeron (1; P. rapensis; Rapa Island),
Paleaepappus (1; P. patagonicus; Patagonia),
Parastrephia (3; P. lucida, P. quadrangularis,
P. teretiuscula; central Andes; in Diplostephium?),
Piofontia (c 60; Costa Rica, the Andes in Colombia, Venezuela and
Ecuador), Pleurophyllum (4; P. criniferum, P.
hookeri, P. oresigenesum, P. speciosum; Campbell
Islands, Macquarie Island and other islands south of New Zealand), Printzia
(6; P. aromatica, P. auriculata, P. huttoni, P.
nutans, P. polifolia, P. pyrifolia; Western and Eastern
Cape, KwaZulu-Natal, Mpumalanga, Free State), Pteronia
(70–75; Africa, especially southwestern South Africa), Remya
(3; R. kauaiensis, R. mauiensis, R. montgomeryi; the
Hawaiian Islands), Rochonia (2; R. cinerarioides, R.
cuneata; Madagascar), Westoniella (6; W. barqueroana,
W. chirripoensis, W. eriocephala, W. kohkemperi,
W. lanuginosa, W. triunguifolia; Costa Rica, Panamá), Brachyscome
(c 80; New Guinea, Australia, Tasmania, New Zealand), Allittia (2;
A. cardiocarpa, A. uliginosa; South Australia, Victoria,
southern New South Wales, Tasmania), Hullsia (1; H.
argillicola; central Northern Territory), Pembertonia (1; P.
latisquamea; westernmost Western Australia), Calotis (27;
Southeast Asia to Australia), Ceratogyne (1; C. obionoides;
southern Australia), Bellis
(12; Europe, the Mediterranean, North Africa), Bellium (4; B.
bellidioides, B. crassifolium, B. minutum, B.
nivale; the Mediterranean), Akeassia (1; A. grangeoides;
tropical Africa), Apodocephala (9; Madagascar), Ceruana (1;
C. pratensis; Egypt, tropical Africa), Colobanthera (1;
C. waterlotii; Madagascar), Cyathocline (4; C.
jacquemontii, C. lutea, C. manilaliana, C.
purpurea; India, the Himalayas, southern China, Indochina),
Dacryotrichia (1; D. robinsonii; Zambia), Dichrocephala
(6; D. auriculata, D. benthamii, D.
chrysanthemifolia, D. gossypina, D. hamiltonii, D.
integrifolia; tropical and southern Africa, Madagascar, tropical Asia),
Egletes (10; Texas, Mexico, Central America, the West Indies, tropical
South America), Erodiophyllum (2; E. acanthocephalum, E.
elderi; southern Australia), Grangea (8; tropical and subtropical
Africa, Madagascar, tropical and subtropical Asia), Grangeopsis (1;
G. perrieri; Madagascar), Grauanthus (2; G.
linearifolius, G. parviflorus; tropical Africa),
Gyrodoma (1; G. hispida; Mozambique), Heteromma (3;
H. decurrens, H. krookii, H. simplicifolium;
mountains in South Africa and Lesotho), Mtonia (1; M.
glandulifera; Tanzania), Nidorella (16; tropical and southern
Africa, Madagascar), Pilbara (1; P. trudgenii; Hamersley
Range in Western Australia), Plagiocheilus (6; P. bogotensis,
P. ciliaris, P. frigidus, P. peduncularis, P.
soliviformis, P. tanacetoides; tropical South America),
Keysseria (5; K. erici, K. helenae, K.
maviensis, K. pickeringii, K. pinguiculiformis; Central
and East Malesia, the Hawaiian Islands), Lagenocypsela (2; L.
latifolia, L. papuana; New Guinea), Lagenophora (18;
India, China, Southeast Asia, Borneo, Japan, Taiwan, Australia, New Zealand,
Guatemala, Chile, Juan Fernández Islands, Argentina), Myriactis (16;
the Caucasus to Japan and New Guinea, Central America), Novaguinea (1;
N. rudalliae; New Guinea), Pappochroma (5; P.
gunnii, P. pappocromum, P. setosum, P.
stellatum, P. uniflorum; southeastern South Australia, Victoria,
southeastern New South Wales, Tasmania), Phacellothrix (1; P.
cladochaeta; East Malesia, Queensland), Piora (1; P.
ericoides; New Guinea), Pytinicarpa (2; P.
neocaledonica, P. sarasinii; New Caledonia), Rhamphogyne
(1; R. rhynchocarpa; Rodrigues), Rhynchospermum (1; R.
verticillatum; East and Southeast Asia), Sheareria (1; S.
nana; China), Solenogyne (3; S. bellioides, S.
dominii, S. gunnii; southeastern South Australia to southeastern
Queensland, Tasmania), Thespis (4; T. divaricata, T.
erecta, T. integrifolia, T. tonkinensis; Southeast
Asia), Welwitschiella (1; W. neriifolia; Angola, Zambia),
Archibaccharis (37; Mexico to central Panamá), Baccharis
(410–420; southern Canada, United States, Mexico, Central America, the West
Indies, South America), Heterothalamus (2; H. alienus, H.
psiadioides; South America), Asteropsis (2; A.
macrocephala, A. megapotamica; southern Brazil, Uruguay),
Camptacra (3; C. barbata, C. brachycomoides, C.
gracilis; New Guinea, northern and eastern Australia),
Dichromochlamys (1; D. dentatifolia; western and central
Australia), Dimorphocoma (1; D. minutula; South Australia,
western New South Wales), Elachanthus (2; E. glaber, E.
pusillus; southern Australia), Exostigma (2; E.
notobellidiastrum, E. rivulare; southern Brazil and Uruguay to
Bolivia, Paraguay and Argentina), Inulopsis (3; I. camporum,
I. scaposa, I. stenophylla; South America),
Iotasperma (2; I. australiensis, I. sessilifolia;
northern and central Australia), Isoetopsis (1; I.
graminifolia; southern Australia, Tasmania), Ixiochlamys (4;
I. cuneifolia, I. filicifolia, I. integerrima,
I. nana; western and central Australia, South Australia),
Kippistia (1; K. suaedifolia; southern Australia),
Laennecia (13; southwestern United States, Mexico, Central America,
tropical South America), Microgyne (2; M. marchesiana, M.
trifurcata; Brazil, Uruguay, Argentina), Minuria (11; Australia),
Peripleura (5; P. bicolor, P. diffusa, P.
hispidula, P. scabra, P. sericea; Australia; in
Vittadinia?), Podocoma (8; Brazil, Argentina),
Sommerfeltia (2; S. cabrerae, S. spinulosa; South
America), Tetramolopium
(38; New Guinea, eastern Queensland, the Cook Islands, the Hawaiian Islands),
Vittadinia (28; New Guinea, Australia, Tasmania, New Caledonia, New
Zealand), Arctogeron (1; A. gramineum; Central Asia,
Mongolia, China), Aster
(c 180; Europe, southeastern Africa, temperate Asia, one species in North
America), Asterothamnus (7; A. alyssoides, A.
centrali-asiaticus, A. fruticosus, A. heteropappoides,
A. molliusculus, A. poliifolius, A. schischkinii;
Central Asia, Mongolia, China), Callistephus
(1; C. chinensis; China), Galatella
(c 30; Europe, temperate Asia), Kemulariella (6; K.
abchasica, K. caucasica, K. colchica, K. rosea,
K. tahirelcii, K. tuganiana; Turkey, the Caucasus to
Azerbaijan), Krylovia (1; K. karataviensis; Central Asia,
China), Miyamayomena (6; M. angustifolia, M.
koraiensis, M. piccolii, M. savatieri, M.
simplex, M. yuanqunensis; East Asia), Psychrogeton (14;
southwestern to Central Asia), Tripolium
(2; T. pannonicum, T. sorrentinois; temperate regions on the
Northern Hemisphere), Acamptopappus (2; A. shockleyi, A.
sphaerocephalus; southwestern United States), Amphiachyris (2;
A. amoena, A. dracunculoides; central United States, Texas),
Amphipappus (1; A. fremontii; southwestern United States),
Bigelowia (5; B. bolanderi, B. intricata, B.
nudata, B. nuttallii, B. viscidiflora; southeastern
United States), Chihuahuana (1; C. purpusii; northern
Mexico), Chrysoma (1; C. pauciflosculosa; southeastern United
States), Chrysothamnus (9; southwestern Canada, western United
States), Columbiadoria (1; C. hallii; northwestern United
States), Eastwoodia
(1; E. elegans; southwestern United States), Euthamia
(6; E. caroliniana, E. graminifolia, E.
gymnospermoides, E. leptocephala, E. minor, E.
occidentalis; Canada, United States, northwestern Mexico),
Gundlachia (9; Texas, Mexico, Central America to Venezuela),
Gutierrezia (32; United States, Mexico, Bolivia, Chile, Argentina),
Gymnosperma (1; G. glutinosum; southwestern United States,
Mexico, Guatemala), Lorandersonia (7; L. baileyi, L.
linifolia, L. microcephala, L. peirsonii, L.
pulchella, L. salicina, L. spathulata; western United
States, northern Mexico), Medranoa (1; M. parrasana; Coahuila
and Zacatecas in Mexico), Neonesomia (1; N. palmeri; Texas,
northwestern Mexico), Nestotus (2; N. macleanii: Yukon
Territory; N. stenophyllus: western United States),
Oreochrysum (1; O. parryi; western United States, northern
Mexico), Petradoria (1; P. pumila; southwestern United
States), Sericocarpus (5; S. asteroides, S.
linifolius, S. oregonensis, S. rigidus, S.
tortifolius; southwestern Canada, United States), Solidago
(c 110; Macaronesia, temperate regions on the Northern Hemisphere, South
America), Stenotus (4; S. acaulis, S. armerioides,
S. lanuginosus, S. pulvinatus; southwestern Canada, western
United States, Baja California), Thurovia (1; T. triflora;
southeastern Texas), Toiyabea (1; T. alpina; southern
Nevada), Xylovirgata (1; X. pseudobaccharis; Mexico), Pentachaeta
(5; P. alsinoides, P. aurea, P. bellidiflora, P.
fragilis, P. lyonii; southwestern United States, northwestern
Mexico), Rigiopappus (1; R. leptocladus; western United
States), Tracyina (1; T. rostrata; California),
Batopilasia (1; B. byei; Mexico), Boltonia
(7; B. lautureana: China, the Korean Peninsula, Japan, the Russian Far
East; B. apalachicolensis, B. asteroides, B.
caroliniana, B. decurrens, B. diffusa, B.
montana: southern Canada, central and eastern United States),
Chloracantha (1; C. spinosa; southern United States, Mexico,
Central America), Benitoa (1; B. occidentalis; California),
Corethrogyne
(2; C. californica, C. filaginifolia; Oregon, California,
Baja California), Grindelia
(c 65; southwestern Canada, central and western United States, Mexico, western
South America), Haplopappus
(c 70; South America, with their largest diversity in Chile; monophyletic?),
Hazardia (11; Oregon, Nevada, California, Baja California),
Herrickia (4; H. glauca, H. horrida, H.
kingii, H. wasatchensis; western United States), Isocoma
(15; southwestern United States, Mexico), Lessingia (10; southwestern
United States, northwestern Mexico), Machaeranthera (27; southwestern
Canada, western United States, Mexico), Arida (9; western United
States, northern Mexico), Dieteria (2; D. bigelovii, D.
canescens; western Canada, Western United States, northwestern Mexico), Xanthisma
(10; southwestern Canada, United States, Mexico), Olivaea (2; O.
leptocarpa, O. tricuspis; Mexico), Oonopsis (4; O.
engelmannii, O. foliosa, O. multicaulis, O.
wardii; central United States), Pyrrocoma
(15; western North America), Rayjacksonia (3; R. annua,
R. aurea, R. phyllocephala; southwestern and southern United
States, northern Mexico), Stephanodoria (1; S. tomentella;
Mexico), Triniteurybia (1; T. aberrans; northwestern United
States), Xanthocephalum (7; X. benthamianum, X.
centauroides, X. durangense, X. eradiatum, X.
gymnospermoides, X. humile, X. megalocephalum;
southwestern United States, Mexico), Xylorhiza (10; western United
States, Mexico), Almutaster (1; A. pauciflorus; western
Canada, western United States, northern and central Mexico),
Ampelaster (1; A. carolinianus; eastern United States),
Canadanthus (1; C. modestus; North America),
Psilactis (5; P. asteroides, P. brevilingulata,
P. gentryi, P. heterocarpa, P. odysseus;
southwestern United States, Mexico, South America), Symphyotrichum
(c 100; East Asia, Canada, United States, northern Mexico),
Chaetopappa (11; southwestern United States, northern Mexico),
Monoptilon (2; M. bellidiforme, M. bellioides;
southwestern United States, northwestern Mexico), Astranthium (12;
southern United States, Mexico), Dichaetophora (1; D.
campestris; southern United States, northern Mexico), Geissolepis
(1; G. suaedifolia; Mexico), Townsendia (26–27; western
Canada, western United States, northern Mexico), Chrysopsis
(12; southeastern United States to Mexico and the Bahamas), Croptilon
(3; C. divaricatum, C. hookerianum, C. rigidifolium;
southeastern United States), Heterotheca
(c 25; United States, Mexico, Belize), Noticastrum (18; tropical South
America), Osbertia (4; O. bartlettii, O.
chihuahuana, O. heleniastrum, O. stolonifera; Mexico,
Guatemala), Pityopsis (6; P. falcata, P. flexuosa,
P. graminifolia, P. oligantha, P. pinifolia, P.
ruthii; eastern United States to Mexico, Central America),
Tomentaurum (2; T. niveum, T. vandevenderorum;
Mexico), Aphanostephus (8; United States, Mexico), Apopyros
(2; A. corymbosus, A. warmingii; southern Brazil, Paraguay,
Argentina), Conyza (150–155; temperate to tropical regions on both
hemispheres), Erigeron
(470–480; cosmopolitan except Australia, with their highest diversity in
North to Central America), Hysterionica (13; southern Brazil, Uruguay,
Argentina), Leptostelma (6; L. camposportoi, L.
catharinense, L. maximum, L. meyeri, L.
tucumanense, L. tweediei; South America), Afroaster (18;
sub-Saharan Africa). – Cosmopolitan, with their largest diversity in North
America, Australia and Africa. Shrubs or herbs. Latex usually absent. Capitula
radiate, disciform or discoid, with multiseriate usually imbricate involucral
bracts. Ray corollas usually strap-shaped, disc corollas quadri- or
quinquelobate, filiform to infundibuliform. Anther thecae ecalcarate and
usually ecaudate. Stylar branches scabrous or plumose, pronate, with sweeping
hairs on acute appendage. Pappus consisting of uni- to quadriseriate barbellate
bristles, or with scales or awns, or absent. x = 9.
Anthemideae Cass. in
J. Phys. Chim. Hist. Nat. Arts 88: 192. Mar 1819
105/1.860–1.880.
Adenanthellum (1; A. osmitoides; Mpumalanga, northern
KwaZulu-Natal, Swaziland), Athanasia
(39; South Africa, one species in Namibia), Hymenolepis
(7; H. cynopus, H. dentata, H. gnidioides, H.
incisa, H. indivisa, H. parviflora, H.
speciosa; Western and Eastern Cape), Cotula
(60–65; Africa, southwestern and southeastern Australia, Tasmania, Mexico to
South America), Leptinella
(33; New Guinea, southwesternmost Western Australia, eastern New South Wales,
Victoria, Tasmania, New Zealand, Macquarie Island, Campbell Island, Auckland
Islands, Antipodes Islands, Tierra del Fuego, Antarctic islands), Soliva
(6; S. anthemifolia, S. macrocephala, S. sessilis,
S. stolonifera, S. triniifolia, S. valdiviana; South
America), Eriocephalus
(c 35; southern Africa), Hilliardia (1; H. zuurbergensis;
Eastern Cape, KwaZulu-Natal), Hippia
(8; H. bolusae, H. frutescens, H. hirsuta, H.
hutchinsonii, H. integrifolia, H. montana, H.
pilosa, H. trilobata; Western and Eastern Cape), Inezia
(2; I. integrifolia, I. speciosa; Northern Province,
Mpumalanga, Swaziland), Inulanthera (10; Angola, Zimbabwe, southern
Africa, Madagascar), Lasiospermum
(4; L. bipinnatum, L. brachyglossum, L. pedunculare,
L. poterioides; Egypt, southern Africa), Lidbeckia
(2–3; L. pectinata, L. pinnata, L. quinqueloba;
Western Cape), Osmitopsis
(9; Western and Eastern Cape), Adenoglossa (1; A. decurrens;
Northern Cape), Leucoptera (3; L. nodosa, L.
oppositifolia, L. subcarnosa; Northern and Western Cape),
Myxopappus (2; M. acutilobus, M. hereroensis;
Namibia, Namaqualand in Northern Cape), Oncosiphon
(9; Namibia, South Africa, Lesotho), 'Foveolina' (4; F.
burchellii, F. dichotoma, F. schinziana, F.
tenella; Namibia, Northern and Western Cape; polyphyletic?; pro parte in
Myxopappus?), 'Pentzia'
(27; Morocco, Algeria, southern Africa; paraphyletic; incl.
Cymbopappus and Marasmodes?), Cymbopappus
(3; C. adenosolen, C. hilliardiae, C. piliferus;
Western and Eastern Cape, Mpumalanga; in Pentzia?),
Marasmodes (13; Western Cape; in Pentzia?),
Eumorphia (6; E. corymbosa, E. davyi, E.
dregeana, E. prostrata, E. sericea, E.
swaziensis; South Africa, Swaziland), Gymnopentzia (1; G.
bifurcata; South Africa, Lesotho), Phymaspermum (c 20; Zimbabwe,
southern Africa), Schistostephium
(13; tropical and southern Africa), Thaminophyllum (3; T.
latifolium, T. multiflorum, T. mundii; Western Cape), Ursinia
(42; Ethiopia, southern Africa), Ajania
(33; Central and East Asia), Ajaniopsis (1; A.
penicilliformis; Tibet, western China), Arctanthemum
(1–2; A. arcticum, A. integrifolium; arctic and subarctic
regions), Brachanthemum (10; Central Asia to China),
Chrysanthemum (c 40; Europe, temperate and Central Asia),
Elachanthemum (1; E. intricatum; China), Hulteniella
(1; H. integrifolia; arctic regions), Phaeostigma (6; P.
purpureum, P. quercifolium, P. ramosum, P.
salicifolium, P. tibeticum, P. variifolium; China),
Tridactylina (1; T. kirilowii; eastern Siberia), 'Artemisia'
(c 550; temperate regions on the Northern Hemisphere, few species in southern
Africa and western South America; non-monophyletic), Crossostephium
(3; C. artemisioides, C. chinense, C. foliosa; East
Asia), Neopallasia (1; N. pectinata; Central Asia to China),
Picrothamnus (1; P. desertorum; western and southwestern
United States), Artemisiella (1; A. stracheyi; the Himalayas
to China), Allardia (2; A. lasiocarpa, A.
transalaica; Central Asia, Tibet, East Asia), Cancrinia (12;
Central Asia, western Himalayas, Tibet, China, Mongolia, Siberia),
Cancriniella (1; C. krascheninnikovii; Central Asia),
Richteria (3; R. djilgense, R. leontopodium, R.
pyrethroides; southwestern to East Asia), Trichanthemis (9;
Central Asia), Ugamia (1; U. angrenica; Central Asia),
Handelia (1; H. trichophylla; Central Asia to China),
Lepidolopsis (1; L. turkestanica; Iran, Afghanistan, Central
Asia), Polychrysum (1; P. tadschikorum; Afghanistan, Central
Asia), Pseudohandelia (1; P. umbellifera; Iran, Afghanistan,
Central Asia, China), Sclerorhachis (3; S. caulescens, S.
platyrachis, S. polysphaera; Afghanistan), Hippolytia
(17; Central Asia to northern China), Kaschgaria (2; K.
brachanthemoides, K. komarovii; Central Asia to western China),
Lepidolopha (8; Central Asia), Leucanthemella
(2; L. linearis, L. serotina; Central and southeastern
Europe), Microcephala (5; M. afghanica, M.
deserticola, M. lamellata, M. subglobosa, M.
turcomanica; Iran, Central Asia), Nipponanthemum
(1; N. nipponicum; Japan), Opisthopappus (2; O.
longilobus, O. taihangensis; northeastern central China),
Stilpnolepis (2; S. centiflora, S. intricata;
Mongolia, China), Tanacetopsis (21; Iran, Central Asia), Achillea
(c 150; Europe, the Mediterranean, temperate Asia, North America), Anacyclus
(12; the Mediterranean), Heliocauta (1; H. atlantica;
Morocco), Leucocyclus (1; L. formosus; Turkey), Anthemis
(175–180; Europe, the Mediterranean to Iran, East Africa), Cota
(c 25; Europe, the Mediterranean), Gonospermum
(5–7; G. canariense, G. ferulaceum, G. fruticosum,
G. gomerae, G. oshanahanii, G. ptarmiciflorum,
G. revolutum; the Canary Islands; in Tanacetum?),
Nananthea (1; N. perpusilla; Corsica, Sardinia), Tanacetum
(165–170; temperate regions on the Northern Hemisphere; incl. Gonospermum?),
Tripleurospermum
(c 40; temperate regions on the Northern Hemisphere), Brocchia (1;
B. cinerea; northern Africa from Mauritania and Mali to Egypt, Sudan
and Ethiopia), Castrilanthemum (1; C. debeauxii; southeastern
Spain), Hymenostemma (1; H. pseudanthemis; Spain, Morocco),
Leucanthemopsis
(7; L. alpina, L. flaveola, L. longipectinata,
L. pallida, L. pectinata, L. pulverulenta, L.
trifurcata; southern Europe, Morocco), Prolongoa (1; P.
hispanica; Spain), Matricaria
(c 25; Europe, temperate Asia, northwestern North America),
Phalacrocarpum (3; P. oppositifolium, P. sericeum,
P. victoriae; northwestern Iberian Peninsula), Xylanthemum
(7; X. fisherae, X. macropodum, X. paghmanense,
X. pamiricum, X. polycladum, X. rupestre, X.
tianschanicum; southern and Central Asia), Aaronsohnia (2; A.
factorovskyi, A. pubescens; North Africa), Argyranthemum
(24; Madeira, the Canary Islands), Glebionis
(2; G. coronaria, G. segetum; Europe, the Mediterranean,
North Africa; incl. Ismelia?), Heteranthemis
(1; H. viscidehirta; southwestern Europe, North Africa),
Ismelia (1; I. carinata; Morocco; in Glebionis?),
Chamaemelum
(3; C. fuscatum, C. mixtum, C. nobile; Europe, the
Canary Islands, the Mediterranean), Cladanthus
(5; C. arabicus, C. eriolepis, C. flahaultii, C.
mixtus, C. scariosus; the Mediterranean, northwestern Africa),
Mecomischus (2; M. halimifolius, M. pedunculatus;
Morocco, Algeria), Rhetinolepis (1; R. lonadioides; Algeria,
Tunisia, Libya), Santolina
(18; the Mediterranean), Daveaua (1; D. anthemoides;
Portugal, Morocco), Endopappus (1; E. macrocarpus; North
Africa), Heteromera (2; H. fuscata, H. philaenorum;
North Africa), Lepidophorum (1; L. repandum; Spain,
Portugal), Chlamydophora (1; C. tridentata; southern Europe,
North Africa), Chrysanthoglossum (2; C. deserticola, C.
trifurcatum; North Africa), Coleostephus
(3; C. multicaulis, C. myconis, C. paludosus;
western Europe, North Africa), Glossopappus (1; G. macrotus;
southwestern Europe, North Africa), Leucanthemum
(c 40; Europe, northern Asia), Plagius (3; P. flosculosus:
the Balearic Islands; P. grandis, P. maghrebinus: Morocco,
Algeria, Tunisia), Rhodanthemum (15; Spain, Morocco, Algeria), Lonas
(1; L. annua; western Mediterranean), Nivellea
(1; N. nivellei; Morocco), Otospermum (1; O.
glabrum; southwestern Europe, northwestern Africa). – Cosmopolitan, with
their highest diversity in southern Africa, Central and East Asia and the
Mediterranean. Shrubs or herbs. Leaves usually alternate. Involucral bracts bi-
to septaseriate, imbricate, with scarious margins and apex. Ray corollas
trilobate, disc corollas uni- to quinquelobate (or lobes absent) to tri- to
sexalobate. Upper part of filaments with thickened cell walls, forming anther
collar (filament collar). Anther thecae usually ecalcarate and ecaudate. Pollen
grains without internal foramina. Stylar branches hairy, with parallel
stigmatic surfaces. Pappus not capillary. x = 9.
[Inuleae+[Athroismeae+[Feddeeae+[Helenieae+[Coreopsideae+[[Neurolaeneae+[Tageteae+[Bahieae+Chaenactideae]]]+[[Polymnieae+[Heliantheae+[Millerieae+[Madieae+[Eupatorieae+Perityleae]]]]]]]]]]]
Inuleae Cass. in J.
Phys. Chim. Hist. Nat. Arts 88: 193. Mar 1819
58/580–585. Duhaldea (10;
East Africa, Iran to the Himalayas), Caesulia (1; C.
axillaris; Sri Lanka, northeastern India, Bangladesh, Nepal, Burma),
Blumea (c 110; tropical and southern Africa, tropical Asia, northern
Australia), Vicoa (2–3; V. indica, V. lignea;
Africa, India to China and Southeast Asia), Merrittia (1; M.
benguetensis; the Philippines), Schizogyne
(3; S. glaberrima, S. obtusifolia, S. sericea; the
Canary Islands), Vieria
(1; V. laevigata; Tenerife in the Canary Islands),
Buphthalmum (2; B. inuloides, B. salicifolium;
Europe, western Asia), Pallenis (6; P. cuspidata, P.
cyrenaica, P. hierochuntica, P. maritima, P.
spinosa, P. teknensis; the Mediterranean), Rhanterium
(3; R. adpressum, R. epapposum, R. suaveolens;
northwestern Africa to Pakistan), Limbarda (2; L.
crithmoides: the British Isles, southwestern Europe, the Mediterranean;
L. salsoloides: Mongolia), 'Pulicaria'
(c 70; Europe, the Mediterranean, North Africa, temperate Asia; polyphyletic),
Jasonia (3; J. longifolia, J. radiata, J.
tuberosa; France, Spain, Morocco), Dittrichia
(2; D. graveolens, D. viscosa; the Mediterranean),
Iphiona (9; northeastern Africa, southwestern to Central Asia),
Monactinocephalus (2; M. paniculatus, M. shirensis;
tropical Africa, Madagascar), Telekia (2; T. speciosa:
central and eastern Europe, the Balkan Peninsula to the Caucasus; T.
speciosissima: northern Italy), Codonocephalum (1; C.
paecockianum; eastern Turkey, Southwest and Central Asia), Inula
(c 40; Europe, the Mediterranean, Africa, temperate Asia),
'Chrysophthalmum' (4; C. dichotomum, C. gueneri,
C. leptocladum, C. montanum; Turkey, Iraq, Iran;
polyphyletic), Carpesium
(c 25; central and southeastern Europe, Asia, eastern Queensland, northeastern
New South Wales), Rhanteriopsis (2; R. lanuginosa, R.
microcephala; Lebanon, Syria, Iran, Iraq), Pentanema (c 40;
Europe, Africa, Southwest, Central and South Asia), Allagopappus
(2; A. canariensis, A. viscosissimus; the Canary Islands),
Chiliadenus (10; western and southern Mediterranean),
Anvillea (2; A. garcinii, A. platycarpa; North
Africa, southwestern Asia), Lifago (1; L. dielsii; Morocco,
Algeria), Asteriscus
(10; Macaronesia, the Mediterranean), Stenachaenium (5; S.
adenanthum, S. campestre, S. macrocephalum, S.
megapotamicum, S. riedelii; southern Brazil, Uruguay, Paraguay,
Argentina), Antiphiona (2; A. fragrans, A.
pinnatisecta; tropical and southwestern Africa to Namibia),
Calostephane (6; C. angolensis, C. divaricata,
C. huillensis, C. madagascariensis, C. marlothiana,
C. punctulata; tropical and southern subtropical Africa, Madagascar),
Pegolettia (9; Africa, the Arabian Peninsula to India),
Geigeria (30; tropical and southern Africa), Ondetia (1;
O. linearis; Namibia), Sachsia (2; S. polycephala,
S. tricephala; Florida, the Bahamas, Cuba), Iphionopsis (1;
I. oblanceolata; northeastern and eastern Africa, Madagascar),
Cratystylis (4; C. centralis, C. conocephala, C.
microphylla, C. subspinescens; arid and semiarid regions in
western and southern Australia), Pterocaulon (20; Southeast Asia,
Malesia, Australia, New Caledonia, tropical and subtropical America),
Cylindrocline (2; C. commersonii, C. lorencei;
Mauritius), Epaltes (9; tropical regions on both hemispheres),
Litogyne (1; L. gariepina; tropical and southern Africa),
Pechuel-loeschea (1; P. leubnitziae; Namibia, South Africa,
Swaziland, Botswana, Zimbabwe), Sphaeromorphaea (1; S.
russelliana; Australia except western and southwestern parts),
Adelostigma (2; A. athrixioides, A. senegalensis;
tropical Africa), Pluchea
(55–60; tropical and subtropical regions on both hemispheres),
Delamerea (1; D. procumbens; northern Kenya),
Neojeffreya (1; N. decurrens; tropical Africa, Madagascar),
Triplocephalum (1; T. holstii; tropical East Africa),
Sphaeranthus (c 35; Egypt, southern Asia from Iran to Java),
’Pseudoblepharispermum’ (2; P. bremeri: Ethiopia; P.
mudugense: Somalia; non-monophyletic), Pseudoconyza (1; P.
viscosa; tropical Africa, tropical Asia, Central America),
Blumeopsis (1; B. flava; India to West Malesia),
Laggera (9; tropical and southern Africa, the Arabian Peninsula,
tropical Asia), Nicolasia (7; N. coronata, N.
costata, N. felicioides, N. heterophylla, N.
nitens, N. pedunculata, N. stenoptera; southwestern
tropical Africa), Doellia (1; D. bovei; tropical Africa,
Madagascar, the Arabian Peninsula), Porphyrostemma (3; P.
chevalieri, P. grantii, P. monocephala; tropical
Africa), Allopterigeron (1; A. filifolius; northern
Australia), Nanothamnus (1; N. sericeus; Mumbay in India).
– Temperate to tropical regions on both hemispheres, few species in America.
Shrubs or herbs. Capitula radiate, disciform or discoid, with imbricate usually
multiseriate involucral bracts. Ray corollas trilobate strap-shaped or absent,
disc corollas quinquelobate. Anther thecae usually ecalcarate and caudate.
Stylar branches with long acute or obtuse sweeping hairs. Cypsela often with
glandular hairs and/or twin hairs; each epidermal cell usually with one
elongated crystal. Phytomelan (phytomelanin) not found. Pappus consisting of
capillary bristles, of bristles and/or scales, of awns, or absent. x = 10.
[Athroismeae+[Feddeeae+[Helenieae+[Coreopsideae+[[Neurolaeneae+[Tageteae+[Bahieae+Chaenactideae]]]+[[Polymnieae+[Heliantheae+[Millerieae+[Madieae+[Eupatorieae+Perityleae]]]]]]]]]]
Athroismeae Panero in
J. L. Panero et V. A. Funk, Proc. Biol. Soc. Washington 115: 917. 20 Dec
2002
9/80–85. Lowryanthus (1;
L. rubens; southeastern Madagascar), Athroisma (12; tropical
regions in the Old World), Blepharispermum (16; Africa, the Arabian
Peninsula to Sri Lanka), Leucoblepharis (1; L. subsessilis;
India), Anisochaeta (1; A. mikanioides; Eastern Cape,
KwaZulu-Natal), Artemisiopsis (1; A. villosa; tropical and
southern Africa), Symphyllocarpus (1; S. exilis; eastern
Siberia, Manchuria), Centipeda (11; Madagascar, tropical Asia,
Australia, islands in the Pacific, Chile), Anisopappus (35–40;
tropical and southern Africa, Madagascar, one species, A. chinensis,
in India, southern China, Burma and northern Thailand). – Tropical and
subtropical regions in the Old World, Chile. Shrubs or herbs. Capitula
sometimes aggregated in pseudocephalia. Capitula radiate, disciform or discoid,
with few rows of imbricate (often reduced) involucral bracts. Ray corollas
tridentate strap-shaped (outer ones often tubular to filiform) or absent, disc
corollas quinquelobate. Anther thecae usually ecalcarate and caudate or
ecaudate. Stylar branches with short obtuse sweeping hairs. Cypsela sometimes
with phytomelan (phytomelanin). Pappus consisting of scales or awns, or absent.
x = 10.
[Feddeeae+[Helenieae+[Coreopsideae+[[Neurolaeneae+[Tageteae+[Bahieae+Chaenactideae]]]+[[Polymnieae+[Heliantheae+[Millerieae+[Madieae+[Eupatorieae+Perityleae]]]]]]]]]
Feddeeae Pruski,
Herrera, Anderb. et Franc.-Ort. in Syst Bot. 33: 199. 26 Feb 2008
1/1. Feddea (1; F.
cubensis; eastern Cuba). – Leaves leathery. Capitula discoid, with
multiseriate involucral bracts with resiniferous duct. Corollas quinquelobate.
Anther thecae ecalcarate and caudate. Style with inconspicuous hairs. Pappus
consisting of uniseriate capillary bristles. n = ?
[Helenieae+[Coreopsideae+[[Neurolaeneae+[Tageteae+[Bahieae+Chaenactideae]]]+[[Polymnieae+[Heliantheae+[Millerieae+[Madieae+[Eupatorieae+Perityleae]]]]]]]]
x = 19.
Helenieae (Cass.)
Lindl. in J. C. Loudon, Encycl. Plant.: 1074. 1829
13/c 130. Balduina (3; B.
angustifolia, B. atrpurpurea, B. uniflora; southeastern
United States), Gaillardia
(c 25; southern Canada, United States, Mexico, temperate South America), Helenium
(c 40; southern Canada, United States, Mexico, northern Central America, Cuba,
Hispaniola, Peru, Uruguay, Chile, Argentina), Marshallia (10; the
United States), Pelucha (1; P. trifida; Mexico),
Plateilema (1; P. palmeri; Texas, Mexico),
Psathyrotes (3; P. annua, P. pilifera, P.
ramosissima; southwestern United States, Mexico), Trichoptilium
(1; T. incisum; southwestern United States, northwestern Mexico),
Amblyolepis (1; A. setigera; Texas, Mexico), Baileya
(3; B. multiradiata, B. pauciradiata, B.
pleniradiata; southwestern United States, Mexico), Hymenoxys
(26; southwestern Canada, western United States, Mexico, Peru, Bolivia,
Paraguay, Uruguay, Argentina), Psilostrophe (7; P. bakeri,
P. cooperi, P. gnaphalodes, P. mexicana, P.
sparsiflora, P. tagetina, P. villosa; western United
States, Mexico), Tetraneuris (9; North America, Mexico). – America,
with their highest diversity in western North America and Mexico. Usually
herbs. Capitula radiate or discoid, with bi- or multiseriate involucral bracts.
Ray corollas trilobate strap-shaped or absent, disc corollas quinquelobate
tubular. Anther thecae ecalcarate and caudate or ecaudate. Stylar branches with
terminal hair tuft. Cypsela cell walls usually with few large crystals.
Phytomelan not found. Pappus consisting of scales or bristles. x = 19.
[Coreopsideae+[[Neurolaeneae+[Tageteae+[Bahieae+Chaenactideae]]]+[[Polymnieae+[Heliantheae+[Millerieae+[Madieae+[Eupatorieae+Perityleae]]]]]]]
Coreopsideae (Cass.)
Lindl. in J. C. Loudon, Encycl. Plant.: 1074. 1829
28/465–475. Chrysanthellum
(11; Mexico, Central America, the West Indies, Galápagos Islands),
Diodontium (1; D. filifolium; northern Australia),
Glossocardia (12; Southeast Asia, Malesia to islands in the Pacific),
Isostigma (c 15; subtropical South America), Trioncinia (1;
T. retroflexa; eastern Queensland), Bidens
(c 250; cosmopolitan), Coreocarpus (6; C. arizonicus, C.
congregatus, C. dissectus, C. insularis, C.
parthenioides, C. sonoranus; southwestern United States, Mexico),
Coreopsis
(c 35; North to South America), Cosmos
(40–45; United States, Mexico, Central America, the West Indies, tropical
South Americ, with their highest diversity in Mexico), Cyathomone (1;
C. sodiroi; Ecuador), Dahlia
(40–45; Mexico, Central America, Colombia), Dicranocarpus (1; D.
parviflorus; southwestern United States, Mexico), Ericentrodea
(6; E. corazonensis, E. davidsmithii, E.
decomposita, E. homogama, E. mirabilis, E.
ramirezii; the Andes), Fitchia (7; F. cordata, F.
cuneata, F. mangarevensis, F. nutans, F.
rapensis, F. speciosa, F. tahitensis; Polynesia),
Goldmanella (1; G. sarmentosa; Central America),
Henricksonia (1; H. mexicana; Mexico), Heterosperma
(6; H. achaetum, H. nanum, H. ovatifolium, H.
pinnatum, H. tenuisectum, H. xanti; southwestern United
States, Mexico and southwards to South America), Hidalgoa (3; H.
ternata, H. uspanapa, H. wercklei; Mexico, Central
America), Leptosyne
(3; L. dissecta, L. parthenioides, L. pinnata;
southwestern United States, northwestern Mexico), Moonia (6; M.
arnottiana, M. ecliptoides, M. heterophylla, M.
moluccana, M. procumbens, M. trichodesmoides; southern
India, Sri Lanka), Narvalina (1; N. domingensis; Hispaniola),
Oparanthus (3; O. albus, O. hivoanus, O.
teikiteetinii; Rapa Island, Marquesas Islands), Petrobium (1;
P. arboreum; St. Helena), Thelesperma
(13; western North America, Mexico, southern South America), Koehneola
(1; K. repens; eastern Cuba), Pinillosia (1; P.
berteroi; Cuba, Hispaniola), Tetraperone (1; T.
bellioides; Cuba), Staurochlamys (1; S. burchellii;
northern Brazil). – Tropical to warm-temperate regions on both hemispheres,
with their highest diversity in America. Shrubs or herbs. Leaves alternate or
opposite. Capitula radiate or discoid, with usually bi- or multiseriate
involucral bracts with resiniferous ducts (striations). Disc corollas quadri-
or quinquelobate. Anther thecae ecalcarate and caudate. Style simple or shortly
bilobate, papillate. Pappus consisting of two to 15 barbed bristles or short
awns. Cypsela walls with phytomelan (phytomelanin; usually with spines or
striations). x = 18.
[[Neurolaeneae+[Tageteae+[Bahieae+Chaenactideae]]]+[[Polymnieae+[Heliantheae+[Millerieae+[Madieae+[Eupatorieae+Perityleae]]]]]]
[Neurolaeneae+[Tageteae+[Bahieae+Chaenactideae]]]
Neurolaeneae Rydb. in
N. Amer. Fl. 33: 46. 15 Sep 1922
6/170–175. Enydra (6; E.
anagallis, E. fluctuans, E. maritima, E.
radicans, E. sessilifolia, E. sessilis; tropical and
subtropical regions on both hemispheres), Heptanthus (7; H.
brevipes, H. cochlearifolius, H. cordifolius, H.
lobatus, H. ranunculoides, H. shaferi, H.
yumuriensis; Cuba), Calea (145–150; Mexico, Central America,
tropical and subtropical South America), Greenmaniella (1; G.
resinosa; Mexico), Neurolaena (12; Mexico, Central America, one
species, N. lobata, also in Florida, the West Indies and tropical
South America), Tyleropappus (1; T. dichotomus; Venezuela).
– Tropical and subtropical regions on both hemispheres, with their highest
diversity in tropical America. Shrubs or herbs. Stem hollow. Leaves alternate
or opposite. Capitula radiate or discoid, with uni- to octaseriate involucral
bracts. Ray corollas very reduced to well developed and tubular or
strap-shaped, usually trilobate (sometimes absent), disc corollas usually
quinquelobate tubular. Anthers usually black, with ecalcarate and ecaudate
thecae. Pollen grains with partial cavea. Stylar branches with terminal hair
tuft. Cypsela walls with phytomelan (phytomelanin). Pappus absent or consisting
of scales, awns or bristles. n = 11.
[Tageteae+[Bahieae+Chaenactideae]]
Tageteae Cass. in J.
Phys. Chim. Hist. Nat. Arts 88: 162. Feb 1819 [‘Tagetineae’]
30/270–285. Clappia (1;
C. suaedifolia; Texas, Mexico), Coulterella (1; C.
capitata; Baja California), Flaveria
(24; southern United States, Mexico, Central and South America, Australia),
Haploesthes (3; H. fruticosa, H. greggii, H.
robusta; western Mexico), Sartwellia (5; S. flaveriae,
S. gypsophila, S. humilis, S. mexicana, S.
puberula; Mexico), Jaumea (7; J. carnosa, J.
chevalieri, J. helenae, J. linearifolia, J.
linearis, J. peduncularis, J. rotundifolia; western
United States, northwestern Mexico, southern South America),
Adenophyllum (c 10; southwestern United States, Mexico, Central
America), Bajacalia (3; B. crassifolia, B. moranii,
B. tridentata; Baja California), Boeberoides (1; B.
grandiflora; Mexico), Chrysactinia (6; C. acerosa,
C. lehtoae, C. luzmariae, C. mexicana, C.
pinnata, C. truncata; Mexico), Dysodiopsis (1; D.
tagetoides; southern central United States), Dyssodia (8; the
United States, Mexico, Guatemala, with their highest diversity in Mexico),
Gymnolaena (3; G. chiapasana, G. oaxacana, G.
serratifolia; Mexico), Harnackia (1; H. bisecta; eastern
Cuba), Hydropectis (3; H. aquatica, H. estradii,
H. stevensii; Mexico), Lescaillea (1; L.
equisetiformis; western Cuba), Leucactinia (1; L.
bracteata; Mexico), Nicolletia (3; N. edwardsii, N.
occidentalis, N. trifida; southwestern United States,
northwestern Mexico), Pectis (90–95; tropical and subtropical
America including the Galápagos Islands), Porophyllum
(25–30; southwestern United States, Mexico, Central America, the West Indies,
tropical South America), Schizotrichia (1; S. jelskii; Peru),
Strotheria (1; S. gypsophila; northern and central Mexico),
Tagetes
(50–55; tropical and subtropical America), Thymophylla
(13; southwestern United States, Mexico), Urbinella (1; U.
palmeri; Mexico), Varilla (2; V. mexicana, V.
texana; Mexico), Arnicastrum (2; A. glandulosum, A.
guerrerense; Mexico), Jamesianthus (1; J. alabamensis;
Alabama), Oxypappus (1; O. scaber; Mexico),
Pseudoclappia (2; P. arenaria, P. watsonii;
southwestern United States, northern Mexico). –Tropical to warm-temperate
America, with their largest diversity in Mexico. Shrubs or herbs. Leaves
alternate or opposite. Capitula usually radiate, with uni- to quinqueseriate
involucral bracts. Ray corollas bi- or trilobate strap-shaped, disc corollas
quinque- or sexalobate of equal size or one or two lobes larger. Anther thecae
ecalcarate and ecaudate. Stylar branches papillate. Cypsela walls with
phytomelan (phytomelanin). Pappus consisting of scales and/or bristles. x =
19.
[Bahieae+Chaenactideae]
Bahieae B. G. Baldwin
in B. G. Baldwin, B. L. Wessa et J. L. Panero, Syst. Bot. 27: 192. 4 Mar
2002
22/c 92. Achyropappus (1;
A. anthemoides; Mexico), Amauriopsis (1; A.
dissecta; western United States, northern Mexico), Apostates (1;
A. rapae; Rapa Island), Bahia
(c 10; southwestern United States, Mexico, Chile), Bartlettia (1;
B. scaposa; Mexico), Chaetymenia (1; C.
peduncularis; Mexico), Chamaechaenactis (1; C. scaposa;
southwestern United States), Espejoa (1; E. mexicana; Mexico,
Central America), Florestina (8; southern United States, Mexico,
Guatemala), Holoschkuhria (1; H. tetramera; northern Peru),
Hymenopappus (13; southern United States, Mexico),
Hymenothrix (6; H. glandulosa, H. greenmanii, H.
loomisii, H. palmeri, H. wislizeni, H.
wrightii; southwestern United States, Mexico), Hypericophyllum
(11; tropical and southern Africa), Loxothysanus (2; L.
pedunculatus, L. sinuatus; eastern Mexico), Palafoxia
(12; southern United States, Mexico), Peucephyllum (1; P.
schottii; southwestern United States, northern Mexico),
Picradeniopsis (2; western and central United States),
Platyschkuhria (2; P. integrifolia, P. ourolepis;
southwestern United States), Psathyrotopsis (3; P.
hintoniorum, P. purpusii, P. scaposa; southwestern
United States, Mexico), Schkuhria
(7; S. degenerica, S. guatemalensis, S. multiflora,
S. pinnata, S. schkuhrioides, S. senecioides, S.
virgata; southern United States, Mexico, Central America, tropical South
America), ‘Bahia’ (c 13; southwestern United States, Mexico,
Chile; paraphyletic; incl. Achyropappus, Holoschkuhria,
Nothoschkuhria, Apostates and Picradeniopsis?),
Achyropappus (1; A. anthemoides; Mexico; in Bahia?),
Holoschkuhria (1; H. tetramera; northern Peru; in
Bahia?), Nothoschkuhria (1; N. degenerica; Bolivia,
northern Argentina; in Bahia?), Apostates (1; A.
rapae; Rapa Island in Austral Islands; in Bahia?),
Picradeniopsis (2; P. oppositifolia, P. woodhousei;
western and central United States; in Bahia?), Thymopsis (2;
T. brittonii, T. thymoides; the West Indies). – Tropical
and southern Africa, Rapa Island, tropical to warm-temperate America, with
their highest diversity in Mexico. Herbs. Leaves alternate or opposite.
Capitula radiate or discoid, with uni- to quadriseriate imbricate involucral
bracts often with hyaline margins. Ray corollas usually bi- or trilobate
strap-shaped or absent, disc corollas quadri- or quinquelobate. Anther thecae
ecalcarate and ecaudate. Stylar branches with short apical hairs. Cypsela walls
with phytomelan (phytomelanin; often striate). Pappus consisting of scales with
usually thickened base or midrib or of fasciculate or hooked bristles. x =
17.
Chaenactideae B. G.
Baldwin in B. G. Baldwin, B. L. Wessa et J. L. Panero in Syst. Bot. 27: 192. 4
Mar 2002
3/21. Dimeresia (1; D.
howellii; western United States), Chaenactis
(19; western North America, Mexico), Orochaenactis (1; O.
thysanocarpha; California). – Western North America, Mexico. Herbs
(rarely somewhat woody). Leaves usually alternate. Capitula discoid, with uni-
or biseriate involucral bracts. Peripheral corollas sometimes zygomorphic.
Anther thecae ecalcarate and ecaudate. Style with short hairs. Cypsela walls
with phytomelan (phytomelanin). Pappus consisting of setose to obovate scales
without thickened base or midribs, sometimes connate at base into caducous
unit. n = 9.
[Polymnieae+[Heliantheae+[Millerieae+[Madieae+[Eupatorieae+Perityleae]]]]]
Tectal spines solid.
Polymnieae (H. Rob.)
Panero in Proc. Biol. Soc. Washington 115: 919. 20 Dec 2002
1/7. Polymnia
(7; P. aspera, P. canadensis, P. cocuyensis, P.
cossatotensis, P. laevigata, P. quichensis, P.
sonchifolia; southeastern Canada, central and eastern United States,
Mexico, Central America, Colombia, Bolivia). – Perennial herbs. Leaves
opposite. Capitula radiate, with bi- or triseriate imbricate involucral bracts.
Ray corollas trilobate (mid-lobe longer and wider), disc corollas quinquelobate
tubular. Anther thecae ecalcarate and ecaudate. Stylar branches with short
hairs. Cypsela walls with phytomelan (phytomelanin). Pappus coroniform or
absent. n = 15.
[Heliantheae+[Millerieae+[Madieae+[Eupatorieae+Perityleae]]]]
Heliantheae Cass. in
J. Phys. Chim. Hist. Nat. Arts 88: 189. Mar 1819
124/1.650–>1.685. Ambrosia
(c 50; North to South America; incl. Xanthium?),
Xanthium
(10; southern Europe, North Africa, the Middle East to India, Mongolia, China,
Southeast Asia, Bolivia, Chile, Argentina; in Ambrosia?),
Dicoria (3; D. argentea, D. calliptera, D.
canescens; southwestern United States, northwestern Mexico),
Euphrosyne (1; E. parthenifolia; Mexico), Hedosyne
(1; H. ambrosiifolia; southwestern United States), Iva
(11; southern Canada, United States, Mexico, the West Indies), Cyclachaena (1; C. xanthiifolia; central United States), Parthenice (1; P.
mollis; Mexico), Parthenium
(14; North America to northern South America, the West Indies),
Chromolepis (1; C. heterophylla; Mexico), Dugesia
(1; D. mexicana; Mexico); Acunniana (1; A.
procumbens; northern Australia), Baltimora (2; B.
geminata, B. recta; Mexico, Central America, the West Indies,
tropical South America), Calyptocarpus (5; C. biaristatus,
C. brasiliensis, C. burchellii, C. vialis, C.
wendlandii; Texas, Mexico, Guatemala), Clibadium (35–40;
southern Mexico, Central America, the West Indies, tropical South America),
Damnxanthodium (1; D. calvum; Mexico), Delilia (3;
D. biflora, D. inelegans, D. repens; southern
Mexico, Central America, Cuba, tropical South America, the Galápagos Islands),
Dimerostemma (c 20; tropical South America), Eleutheranthera
(3; E. divaricata, E. ruderalis, E. tenella;
tropical America), Exomiocarpon (1; E. madagascariense;
Madagascar), Fenixia (1; F. pauciflora; the Philippines),
Hoffmanniella (1; H. silvatica; Central Africa, Cameroon),
Idiopappus (1; I. saloyensis; Ecuador), Indocypraea
(1; I. montana; India to southern China and West Malesia),
Iogeton (1; I. nowickeanus; Panamá), Jefea (5;
J. brevifolia, J. gnaphalioides, J. lantanifolia,
J. phyllocephala, J. pringlei; southern United States,
Mexico, Guatemala), Kingianthus (2; K. paniculatus, K.
sodiroi; Ecuador), Lantanopsis (3; L. hispidula, L.
hoffmanni, L. tomentosa; Cuba, Hispaniola), Lasianthaea
(14; southwestern United States, Mexico), Leptocarpha (1; L.
rivularis; Chile), Lipoblepharis (5; L. asperrima,
L. floribunda, L. stenophylla, L. thailandica,
L. urticifolia; India and Southeast Asia to China, Japan and Vanuatu),
Monactis (6; M. dubia, M. flaverioides, M.
hieronymi, M. holwayae, M. macbridei, M.
pallatangensis; tropical South America), Oblivia (3; O.
ceronii, O. mikanioides, O. simplex; Panamá,
northwestern tropical South America), Otopappus (17; Mexico, Central
America, the West Indies), Pascalia (1; P. glauca; South
America), Pentalepis (2; P. ecliptoides, P.
tricodesmoides; northwestern Australia), Plagiolophus (1; P.
millspaughii; Yucatán in southern Mexico), Podanthus
(2; P. mitiqui, P. ovatifolius; Chile, Argentina),
Quadribractea (1; Q. moluccana; eastern Indonesia to Timor),
Rensonia (1; R. salvadorica; El Salvador),
Schizopsera (1; S. peduncularis; Ecuador), Synedrella
(1; S. nodiflora; Florida, Mexico, Central America, the West Indies,
tropical South America), Synedrellopsis (1; S. grisebachii;
Argentina), Trigonopterum (1; T. laricifolium; the Galápagos
Islands), Tuberculocarpus (1; T. ruber; Venezuela),
Tuxtla (1; T. pittieri; Mexico), Wamalchitamia (5;
W. appressipila, W. aurantiaca, W. dionysi, W.
strigosa, W. williamsii; Central America), Eclipta
(9–11; northern India, Nepal, China, Japan, Australia, North America to
tropical South America, the West Indies), Lundellianthus (9; Central
America), Sphagneticola (5; S. brachycarpa, S.
calendulacea, S. gracilis, S. trilobata, S.
ulei; India and Sri Lanka to China and Japan, Southeast Asia, Malesia,
Central America, the West Indies, tropical South America),
‘Wedelia’ (105–110; tropical and subtropical Africa, Madagascar,
tropical America; non-monophyletic), Zexmenia (11; Central America),
Elaphandra (14; tropical America), Oyedaea (c 20; Panamá,
northwestern tropical South America to Guyana and Bolivia),
Steiractinia (15; Colombia, Venezuela, Ecuador), Blainvillea
(9–10; Cape Verde, tropical and southern Africa, Yemen, tropical Australia,
Mexico, tropical South America, Galápagos Islands), Riencourtia (5;
R. latifolia, R. longifolia, R. oblongifolia, R.
pedunculosa, R. tenuifolia; northern South America),
Perymeniopsis (1; P. ovalifolia; Mexico), Tilesia
(3; T. baccata, T. macrocephala, T. spilanthoides;
Cuba, tropical South America), Perymenium (c 50; Mexico, Central
America, the Andes in Colombia to Peru), Echinocephalum (1; E.
latifolium; Brazil, Paraguay, Uruguay), Lipotriche (c 15;
tropical Africa to Namibia, Madagascar?), Melanthera (c 30; warmer
regions of United States, Mexico, the West Indies), Apowollastonia (8;
Malesia to New Guinea, Australia), Wollastonia (c 20; East Africa,
Indian Ocean islands, India and Sri Lanka to Southeast Asia, Malesia, China,
Japan, northern and eastern Australia, islands in the Pacific from New
Caledonia and Fiji to Micronesia and the Hawaiian Islands), Lipochaeta
(20; the Hawaiian Islands); Encelia
(20; southwestern United States, Mexico, the Galápagos Islands, Peru, Chile),
Enceliopsis (3; E. argophylla, E. covillei, E.
nudicaulis; western United States), Flourensia (30–35; southern
United States, Mexico, Peru, Chile, Argentina), Geraea
(2; G. canescens, G. viscida; southwestern United States,
northwestern Mexico), Helianthella
(10; southwestern Canada, western United States, Mexico), Agnorhiza
(5; A. bolanderi, A. elata, A. invenusta, A.
ovata, A. reticulata; Sierra Nevada in California),
Balsamorhiza (13; southwestern Canada, western United States),
Berlandiera (8; southern United States, Mexico), Borrichia
(3; B. arborescens, B. frutescens, B. peruviana;
southeastern United States, the West Indies), Chrysogonum (2; C.
perrieri, C. virginianum; southeastern United States),
Engelmannia (2; E. peristenia, E. pinnatifida;
central and southern United States, Mexico), Lindheimera
(2; L. mexicana, L. texana; southern United States, Mexico),
Scabrethia (1; S. scabra; western United States), Silphium
(17; southeastern Canada, United States, Mexico), Vigethia (1; V.
mexicana; Mexico), Wyethia (11; western United States,
northwestern Mexico), Aldama (2; A. dentata, A.
mesoamericana; Central America, tropical South America), Alvordia
(4; A. brandegeei, A. congesta, A. fruticosa, A.
glomerata; California, Mexico), Bahiopsis (10; southwestern
United States, northwestern Mexico), Calanticaria (4; C.
bicolor, C. brevifolia, C. greggii, C. inegii;
southern United States, Mexico), Helianthus
(c 70; southern Canada, United States, northern Mexico, H.
membranifolius and H. sarmentosus in French Guiana, H.
navarri in Chile), Heliomeris (5–8; H. hispida, H.
longifolia, H. multiflora, H. obscura, H.
soliceps; western United States, northern Mexico), Hymenostephium
(8; Mexico, Central America, tropical South America to Argentina),
Iostephane (4; I. heterophylla, I. madrensis, I.
papposa, I. trilobata; Mexico), Lagascea (8; Mexico,
Central America), Pappobolus (23; Colombia, Ecuador, Peru, Bolivia),
Phoebanthus (2; P. grandiflorus, P. tenuifolius;
southeastern United States), Rhysolepis (2; R. kingii, R.
morelensis; Mexico), Scalesia
(10; the Galápagos Islands), Sclerocarpus (11; tropical and southern
Africa, Mexico, Central America), Simsia (c 25; southern United
States, Mexico, Central America, tropical South America), Stuessya (2;
S. apiculata, S. perennans; Mexico), Syncretocarpus
(1; S. sericeus; Peru), Tithonia
(c 20; southwestern United States, Mexico, Central America south to Costa
Rica), Viguiera
(150–160; tropical and subtropical America), Dendroviguiera (14;
Mexico, Central America, tropical South America), Montanoa (c 50;
Mexico, Central America, northern tropical South America), Rojasianthe
(1; R. superba; Central America), Ratibida
(7; R. coahuilensis, R. columnifera, R.
latipalearis, R. mexicana, R. peduncularis, R.
pinnata, R. tagetes; southern Canada, United States, northern
Mexico), Rudbeckia
(c 25; southern Canada, United States), Acmella (c 30; tropical
regions on both hemispheres), Oxycarpha (1; O. suaedifolia;
Venezuela), Salmea (10–12; Mexico, Central America, the West Indies,
tropical South America), Spilanthes
(c 35; tropical regions on both hemispheres), Tetranthus (4; T.
bahamensis, T. cupulatus, T. hirsutus, T.
littoralis; the West Indies), Podachaenium (5; P.
chiapanum, P. eminens, P. pachyphyllum, P.
paniculatum, P. skutchii; Mexico, Costa Rica),
Squamopappus (1; S. skutchii; southeastern Mexico,
Guatemala), Tetrachyron (10; eastern Mexico, Guatemala), Verbesina
(>300; southeastern Canada, United States, Mexico, Central America, the West
Indies, tropical South America to Argentina), Hybridella (1; H.
globosa; Mexico), Zaluzania (14; southwestern United States,
Mexico), Echinacea
(5–10; eastern United States), Heliopsis
(c 15; southeastern Canada, United States, Mexico, Central America,
northwestern tropical South America, with their highest diversity in Mexico),
Philactis (3; P. fayi, P. nelsonii, P.
zinnioides; Central America), Sanvitalia
(7; S. acapulcensis, S. albertii, S. angustifolia,
S. fruticosa, S. ocymoides, S. procumbens, S.
versicolor; southwestern United States, Mexico, Central America),
Tehuana (1; T. calzadae; Mexico), Trichocoryne (1;
T. connata; Mexico), Zinnia
(22; United States, Mexico, Central and South America to Peru, with their
highest diversity in Mexico). – Temperate to tropical America, few species in
the Old World. Trees, shrubs, lianas or herbs. Leaves alternate or opposite.
Capitula radiate or discoid, with uni- to septaseriate often foliaceous
involucral bracts. Receptacular paleae enclosing cypsela and usually
persistent. Ray corollas usually trilobate strap-shaped or absent, disc
corollas quinquelobate. Anther thecae usually black, usually ecalcarate and
ecaudate. Stylar branches often with tuft of hairs or papillae. Cypsela walls
with phytomelan (phytomelanin; often with spines, sometimes with striation).
Pappus consisting of awns or scales (rarely absent). x = 19.
[Millerieae+[Madieae+[Eupatorieae+Perityleae]]]
Millerieae (Cass.)
Lindl. in J. C. Loudon, Encycl. Plant.: 1074. 1829
32/c 430. Desmanthodium (8;
Central and South America), Bebbia (2; B. atriplicifolia,
B. juncea; southwestern United States, northwestern Mexico),
Dyscritothamnus (2; D. filifolius, D. mirandae;
Mexico), Tetragonotheca (2; T. repanda, T. texana;
southeastern United States), Tridax
(c 30; tropical to warm-temperate regions in America, with their highest
diversity in Mexico), Espeletia (c 125?; the Andes in Colombia,
Venezuela, Ecuador), Tamananthus (1; T. crinitus; Venezuela),
Alepidocline (4; A. annua, A. breedlovei, A.
macdonaldana, A. trifida; Mexico, Central America),
Alloispermum (16; Mexico, Central America, tropical South America),
Aphanactis (11; Oaxaca in Mexico, Colombia, Ecuador, Peru, Bolivia),
Cuchumatanea (1; C. steyermarkii; Guatemala),
Faxonia (1; F. pusilla; Baja California), Galinsoga
(15; Mexico, Central America, subtropical and temperate South America),
Oteiza (4; O. acuminata, O. mixtecana, O.
ruacophila, O. scandens; Mexico, Guatemala), Sabazia
(16; mountains in Central America), Schistocarpha (13; southern
Mexico, Central America, western tropical South America), Selloa (6;
S. breviligulata, S. ligulata, S. linearis, S.
macdonaldii, S. obtusata, S. plantaginea; Mexico,
Central America), Guardiola (14; Mexico), Jaegeria (11;
Mexico, Central America, tropical South America to Uruguay, the Galápagos
Islands), Acanthospermum (6; A. australe, A.
consobrinum, A. glabratum, A. hispidum, A.
humile, A. microcarpum; Central America, tropical South America),
Lecocarpus
(2; L. lecocarpoides, L. pinnatifidus; the Galápagos
Islands), Melampodium
(c 45; southwestern United States, Florida, Mexico, Central America, the West
Indies, tropical South America, with their highest diversity in Mexico),
Axiniphyllum (6; A. corymbosum, A. durangense,
A. pinnatisectum, A. sagittalobum, A. scabrum,
A. tomentosum; Mexico), Guizotia
(6; G. abyssinica, G. arborescens, G. jacksonii,
G. scabra, G. schimperi, G. villosa; tropical and
subtropical Africa), Ichthyothere (24; tropical South America),
Micractis (3; M. bojeri, M. discoidea, M.
drosocephala; tropical Africa, Madagascar), Milleria (2; M.
perfoliata, M. quinqueflora; Mexico, Central America to Peru),
Rumfordia (7; R. alcortae, R. connata, R.
exauriculata, R. floribunda, R. guatemalensis, R.
penninervis, R. revealii; Mexico, Central America), Sigesbeckia
(c 20; tropical Africa, tropical Asia), Smallanthus (c 20; tropical
and subtropical America), Stachycephalum (2; S. mexicanum:
Mexico; S. argentinum: Argentina), Trigonospermum (6; T.
adenostemmoides, T. annuum, T. auriculatum, T.
hintoniorum, T. melampodioides, T. stevensii; Mexico).
– Tropical and subtropical Africa, Madagascar, tropical Asia, tropical to
warm-temperate America, with their highest diversity in Mexico, Central America
and northern South America. Trees, shrubs or herbs. Leaves usually opposite,
often glandular. Capitula usually radiate, with uni- to quinqueseriate
involucral bracts. Ray corollas usually trilobate strap-shaped, disc corollas
quadri- or quinquelobate. Anther thecae usually black, ecalcarate and ecaudate.
Stylar branches with short hairs. Cypsela walls with phytomelan (phytomelanin;
with spines and striation). Pappus consisting of scales or bristles, or absent.
x = 19.
[Madieae+[Eupatorieae+Perityleae]]
Madieae Jeps., Fl. W.
Middle Calif.: 483, 486. 1901
34/205–220. Arnica
(30–35; temperate and arctic-alpine regions on the Northern Hemisphere),
Amblyopappus (1; A. pusillus; California, northwestern
Mexico, Peru, Chile), Baeriopsis (1; B. guadalupensis; Baja
California), Constancea (1; C. nevinii; California), Eriophyllum
(13; western North America, Mexico), Lasthenia
(19; southwestern Canada, western United States, northern Mexico, one species,
L. kunthii, in Chile), Monolopia (5; M. congdonii,
M. gracilens, M. lanceolata, M. major, M.
stricta; California), Pseudobahia (3; P. bahiifolia,
P. heermannii, P. peirsonii; California),
Syntrichopappus (2; S. fremontii, S. lemmonii;
southwestern United States), Eatonella (1; E. nivea; western
United States), Hulsea (7; H. algida, H. brevifolia,
H. californica, H. heterochroma, H. mexicana, H.
nana, H. vestita; western United States), Achyrachaena
(1; A. mollis; western North America), Adenothamnus (1;
A. validus; Baja California), Anisocarpus (2; A.
madioides, A. scabridus; western North America, northwestern
Mexico), Blepharipappus (3; B. carnosa, B.
fremontii, B. scaber; western United States),
Blepharizonia (2; B. laxa, B. plumosa; California),
Calycadenia (10; western United States), Carlquistia (1;
C. muirii; California), Centromadia (4; C. fitchii,
C. parryi, C. perennis, C. pungens; California,
northwestern Mexico), Deinandra (21; southwestern United States,
northwestern Mexico), Dubautia
(28; the Hawaiian Islands), Harmonia (5; H. doris-nilesiae,
H. guggolziorum, H. hallii, H. nutans, H.
stebbinsii; California), Hemizonella (1; H. minima;
western United States), Hemizonia (1 or 12; H. congesta;
southwestern United States, northwestern Mexico), Holocarpha (4;
H. heermannii, H. macradenia, H. obconica, H.
virgata; California), Holozonia (1; H. filipes; western
United States), Jensia (2; J. rammii, J. yosemitana;
California), Kyhosia (1; K. bolanderi; Oregon, California),
Lagophylla (4; L. dichotoma, L. glandulosa, L.
minor, L. ramosissima; California to Washingtonwestern North
America), Layia
(19; western United States, northwestern Mexico, with their highest diversity
in California), Madia
(11–12; southwestern Canada, western United States, northwestern Mexico, one
species, M. sativa, also in eastern Canada, northastern United States,
southern Chile, southern Argentina), Osmadenia (1; O.
tenella; California, northwestern Mexico), Raillardella (3;
R. argentea, R. pringlei, R. scaposa; western United
States), Venegasia
(1; V. carpesioides; California, northwestern Mexico). – Temperate
and arctic-alpine regions on the Northern Hemisphere, the Hawaiian Islands,
Mexico, Peru, Chile, with their highest diversity in western United States and
the Hawaiian Islands. Trees, shrubs, lianas or herbs. Leaves alternate,
opposite or verticillate, often glandular. Capitula radiate or discoid, with
usually uni- or biseriate subequal involucral bracts. Ray corollas usually
(deeply) trilobate strap-shaped, disc corollas quinquelobate. Anther thecae
ecalcarate and ecaudate. Style simple to deeply bilobate, branches with short
hairs. Cypsela walls with phytomelan (phytomelanin; with spines or striation).
Pappus consisting of scales or bristles, or absent. x = 19.
[Eupatorieae+Perityleae]
Eupatorieae Cass. in
J. Phys. Chim. Hist. Nat. Arts 88: 202. Mar 1819
168/2.530–2.575.
Hofmeisteria (12; Mexico), Ageratina
(330–340; eastern United States, Central and western South America),
Oxylobus (6; O. adscendens, O. arbutifolius, O.
glanduliferus, O. oaxacanus, O. preecei, O.
subglabrus; Mexico, Central America, Colombia to Venezuela),
Jaliscoa (3; J. goldmanii, J. paleacea, J.
pringlei; Mexico), Spaniopappus (5; S. buchii, S.
ekmanii, S. hygrophilus, S. iodostylus, S.
shaferi; Cuba), Standleyanthus (1; S. triptychus; Costa
Rica), Kaunia (12; South America), Jaramilloa (2; J.
hylibates, J. sanctae-martae; northern Colombia),
Mikania (430–435; tropical regions on both hemispheres),
Trichocoronis (2; T. sessilifolia, T. wrightii;
southwestern United States, northern Mexico), Sclerolepis (1; S.
uniflora; eastern United States), Adenostemma (27; tropical
Africa, Central and tropical South America, the West Indies),
Sciadocephala (5; S. amazonica, S. asplundii, S.
dressleri, S. pakaraimae, S. schultze-rhonhofiae;
Panamá, northern tropical South America), Gymnocoronis (5; G.
latifolia, G. matudae, G. nutans, G. sessilis,
G. spilanthoides; Central America, tropical South America),
Fleischmannia (c 100; southern Canada, United States, Mexico, western
South America), Sartorina (1; S. schultzii; ‘tropical
America’), Acritopappus (16–17; eastern Brazil),
Radlkoferotoma (3; R. berroi, R. cistifolia, R.
ramboi; southern Brazil, Uruguay), Ageratum
(c 50; Central America, the West Indies, tropical South America),
Phania (6; P. trinervia: Mexico; P. domingensis:
Hispaniola; P. cajalbanica, P. curtissii, P.
matricarioides, P. multicaulis: Cuba), Phalacraea (4;
P. callitriche, P. ecuadorensis, P. latifolia,
P. longipetiolata; Colombia, Ecuador, Peru), Blakeanthus (1;
B. cordatus; Guatemala, Honduras), Scherya (1; S.
bahiensis; eastern Brazil), Ascidiogyne (2; A.
sanchezvegae, A. wurdackii; Peru), Cavalcantia (2;
C. glomerata, C. percymosa; Brazil), Teixeiranthus
(2; T. foliosus, T. pohlii; Brazil), Gardnerina (1;
G. angustata; Brazil), Ellenbergia (1; E.
glandulata; Peru), Guevaria (5; G. alvaroi, G.
loxensis, G. micranthera, G. sodiroi, G.
vargasii; Ecuador, Peru), Ferreyrella (2; F.
cuatrecasasii, F. peruviana; Peru), Nesomia (1; N.
chiapensis; Mexico), Piqueriella (1; P. brasiliensis;
Brazil), Piqueria (9; tropical regions on both hemispheres), Stevia
(240–260 [agamospermy]; western United States, Mexico, Central America, the
West Indies, tropical South America), Carphochaete (6; C.
durangensis, C. grahamii, C. gummifera, C.
pringlei, C. schaffneri, C. wislizeni; southwestern
United States, Mexico), Macvaughiella (2; M. chiapensis,
M. mexicana; southern Mexico, Central America), Microspermum
(9; Central America), Eupatorium
(c 40; Europe, temperate Asia, eastern North America),
Austroeupatorium (16; tropical South America to Uruguay), Eutrochium
(5; E. dubium, E. fistulosum, E. maculatum, E.
purpureum, E. steelei; southern Canada, central and eastern
United States), Stomatanthes (17; tropical and southern Africa,
Brazil, Uruguay), Criscianthus (1; C. zambiensis; southern
tropical Africa), Hatschbachiella (2; H. polyclada, H.
tweedieana; southern Brazil), Garberia (1; G.
heterophylla; southeastern United States), Liatris
(c 50; southern Canada, the United States, Mexico, Bahamas),
Carphephorus (7; C. bellidifolius, C. coridifolius,
C. corymbosus, C. odoratissimus, C. paniculatus,
C. pseudoliatris, C. tomentosus; southeastern United States),
Hartwrightia (1; H. floridana; Georgia, Florida),
Praxelis (13–14; tropical South America), Chromolaena (c
175; southern United States, Mexico, Central America, the West Indies, tropical
South America), Eupatoriopsis (1; E. hoffmanniana; Brazil),
Lomatozona (4; L. andersonii, L. artemisiaefolia,
L. huntii, L. inaequale; Brazil), Praxeliopsis (1;
P. mattogrossensis; Brazil), Eitenia (2; E.
polyseta, E. praxeloides; Brazil), Osmiopsis (1; O.
plumieri; Haiti), Gyptis (7; G. artemisifolia, G.
commersonii, G. crassipes, G. inornata, G.
lanigera, G. pinnatifida, G. vernoniopsis; tropical
South America), Gyptidium (2; G. militare, G.
richobasis; Brazil, Argentina), Urolepis (1; U.
hecatantha; subtropical South America), Barrosoa (11; tropical
South America), Dasycondylus (8; Brazil), Diacranthera (3;
D. crenata, D. hebeclinia, D. ulei; Brazil),
Conocliniopsis (1; C. prasiifolia; Colombia, Venezuela,
Brazil), Bejaranoa (2; B. balansae, B. semistriata;
Brazil), Prolobus (1; P. nitidulus; Bahia in Brazil),
Trichogonia (30–35; Brazil, Bolivia, Paraguay),
Trichogoniopsis (4; T. adenantha, T. grazielae,
T. morii, T. podocarpa; Brazil), Platypodanthera (1;
P. melissifolia; eastern Brazil), Neocuatrecasia (12; Peru,
Bolivia), Vittetia (2; V. bishopii, V. orbiculata;
Brazil), Campuloclinium (17; Brazil, Paraguay, Argentina),
Macropodina (3; M. blumenavii, M. bradei, M.
reitzii; southern Brazil, Argentina), Conoclinium (4; C.
betonicifolium, C. coelestinum, C. greggii, C.
mayfieldii; eastern United States, Mexico), Paneroa (1; P.
stachyofolia; Oaxaca in Mexico), Tamaulipa (1; T.
azurea; Texas, northern Mexico), Lourteigia (11; Colombia,
Venezuela), Agrianthus (9; Brazil), Catolesia (2; C.
huperzioides,C. mentiens; Bahia in Brazil),
Arrojadocharis (2; A. praxeloides, A. santosii;
eastern Brazil), Bahianthus (1; B. viscosus; northeastern
Brazil), Semiria (1; S. viscosa; Bahia in Brazil),
Lasiolaena (7; L. blanchetii, L. carvalhoi, L.
duartei, L. lychnophorioides, L. morii, L.
pereirae, L. santosii; eastern Brazil), Stylotrichium
(5; S. corymbosum, S. edmundoi, S. glomeratum,
S. rotundifolium, S. sucrei; eastern Brazil),
Bishopiella (1; B. elegans; eastern Brazil),
Litothamnus (2; L. ellipticus, L. nitidus; Brazil),
Morithamnus (2; M. crassus, M. ganophyllus; eastern
Brazil), Acanthostyles (2; A. buniifolius, A.
saucechicoensis; eastern South America), Raulinoreitzia (3;
R. crenulata, R. leptophlebia, R. tremula; eastern
South America), Disynaphia (16; South America),
Campovassouria (2; C. barbosae, C. cruciata;
Brazil), Grazielia (10; South America), Symphyopappus (13;
Brazil), Ayapana (17; Central America, the West Indies, tropical South
America), Ayapanopsis (18; the Andes), Polyanthina (1; P.
nemorosa; Costa Rica, the Andes from Colombia to Bolivia),
Gongrostylus (2; G. costaricensis, G. pipolyi;
Central America), Heterocondylus (13; Central and South America),
Condylidium (2; C. cuatrecasasii, C. iresinoides;
Central America, the West Indies, Colombia and Venezuela to Bolivia),
Gymnocondylus (1; G. galeopsifolius; Brazil),
Alomiella (2; A. hatschbachii, A. regnellii;
Brazil), Siapaea (1; S. liesneri; Venezuela),
Monogereion (1; M. carajensis; northeastern Brazil),
Parapiqueria (1; P. cavalcantei; Brazil), Lepidesmia
(1; L. squarrosa; Cuba, Colombia, Venezuela), Isocarpha (5;
I. atriplicifolia, I. fistulosa, I. megacephala,
I. microcephala, I. oppositifolia; southern Texas, Mexico,
Central America, the West Indies, tropical South America), Brickellia
(110–115; western United States, Mexico, Central America, tropical South
America to Argentina), Brickelliastrum (1; B. fendleri; New
Mexico, northern Mexico), Flyriella (6; F. chrysostyla,
F. harrimanii, F. leonensis, F. parryi, F.
sphenopoda, F. stanfordii; Texas, northern Mexico),
Ageratella (2; A. microphylla, A. palmeri; Mexico),
Asanthus (3; A. solidaginifolius, A. squamulosus,
A. thyrsiflorus; southwestern United States, Mexico),
Malperia (1; M. tenuis; California, northwestern Mexico),
Pleurocoronis (3; P. gentryi, P. laphamioides,
P. pluriseta; western United States, northwestern Mexico),
Alomia (5; A. ageratoides, A. alata, A.
callosa, A. hintonii, A. stenolepis; Mexico),
Kyrsteniopsis (4; K. congesta, K. cymulifera, K.
dibollii, K. nelsonii; Mexico), Steviopsis (10;
southwestern United States, Mexico), Carminatia (3; C.
papagayana, C. recondita, C. tenuiflora; southwestern
United States, Mexico, Central America to El Salvador), Dissothrix (1;
D. imbricata; Brazil), Austrobrickellia (3; A.
arnottii, A. bakerianum, A. patens; northern tropical
South America), Pseudobrickellia (3; P. angustissima, P.
brasiliensis, P. irwinii; Brazil), Goyazianthus (1;
G. tetrastichus; Brazil), Leptoclinium (1; L.
trichotomum; Brazil), Planaltoa (2; P. lychnophoroides,
P. salviifolia; Brazil), Crossothamnus (4; C.
gentryi, C. killipii, C. pascoanus, C.
weberbaueri; Peru), Helogyne (8; the Andes),
Condylopodium (6; C. cuatrecasasii, C. fuliginosum,
C. gachalanum, C. hyalinifolium, C. killipii, C.
pennellii; Colombia), Critonia (c 45; Mexico, Central America,
the West Indies, tropical South America), Critoniadelphus (2; C.
microdon, C. nubigenus; Central America), Urbananthus
(2; U. critoniformis, U. pluriseriatus; Cuba, Jamaica),
Adenocritonia (3; A. heathiae: Chiapas in Mexico; A.
styermarkii: Guatemala; A. adamsii: Jamaica), Antillia
(1; A. brachychaeta; Cuba), Ciceronia (1; C.
chaptalioides; eastern Cuba), Eupatorina (1; E.
sophiifolia; Hispaniola), Fleischmanniopsis (5; F.
anomalochaeta, F. langmaniae, F. leucocephala, F.
mendax, F. nubigenoides; Mexico, Central America),
Viereckia (1; V. tamauilpasensis; Mexico)?,
Koanophyllon (c 125; southern United States, Mexico, Central America,
the West Indies, tropical South America), Eupatoriastrum (6; E.
angulifolium, E. chlorostylum, E. corvii, E.
nelsonii, E. pochutlanum, E. triangulare; Mexico,
Central America), Sphaereupatorium (1; S. scandens; Brazil,
Bolivia), Bishovia (2; B. boliviensis, B.
mikaniifolia; Bolivia, Argentina), Nothobaccharis (1; N.
candolleana; Peru), Santosia (1; S. talmonii; eastern
Brazil), Grisebachianthus (8; eastern Cuba), Lorentzianthus
(1; L. viscidus; Bolivia, Argentina), Chacoa (1; C.
pseudoprasiifolia; Paraguay, Argentina), Idiothamnus (4; I.
clavisetus, I. lilloi, I. orgyaloides, I.
pseudorgyalis; South America), Mexianthus (1; M.
mexicanus; Mexico), Peteravenia (5; P. cyrilli-nelsonii,
P. grisea, P. malvifolia, P. phoenicolepis, P.
schultzii; Mexico, Central America), Critoniella (6; C.
acuminata, C. albertosmithii, C. lebrijensis, C.
leucolithogena, C. tenuifolia, C. vargasiana; northern
Andes), Aristeguietia
(c 20; the Andes), Asplundianthus (11; northern Andes),
Austrocritonia (4; A. angulicaulis, A. rosea, A.
taunayana, A. velutina; Brazil), Badilloa (10; northern
Andes), Grosvenoria (7; G. campii, G. coelocaulis,
G. hypargyra, G. jelskii, G. lopezii, G.
rimbachii, G. zamorensis; the Andes), Corethamnium (1;
C. chocoense; Colombia), Castenedia (1; C.
santamartensis; Colombia), Imeria (2; I. memorabilis,
I. serratifolia; Venezuela), Malmeanthus (3; M.
catharinensis, M. hilarii, M. subintegerrimus; Brazil,
Uruguay, Argentina), Hughesia (1; H. reginae; Peru),
Ophryosporus (c 40; South America), Cronquistianthus (c 25;
northern Andes), Steyermarkina (4; S. dispalata, S.
dusenii, S. pyrifolia, S. triflora; Venezuela, Brazil),
Neocabreria (4; N. catharinensis, N. malachophylla,
N. pennivenia, N. serrulata; tropical South America),
Uleophytum (1; U. scandens; Peru), Amboroa (2;
A. geminata, A. wurdackii; Peru, Bolivia),
Tuberostylis (2; T. axillaris, T. rhizophorae;
Panamá, Colombia), Hebeclinium (c 25; southern Mexico, Central
America, tropical South America), Amolinia (1; A. heydeana;
Mexico, Guatemala), Bartlettina
(c 40; southern Mexico, Central America, tropical South America),
Decachaeta (8; Mexico, Guatemala), Guayania (6; G.
bulbosa, G. cerasifolia, G. crassicaulis, G.
penninervata, G. roupalifolia, G. yaviana; the Guayana
Highlands), Neomirandea (c 30; Mexico, Central America, northern South
America to Ecuador). – America, few species in the Old World. Trees, shrubs
or herbs. Leaves alternate or opposite. Capitula discoid, with various,
persistent to caducous, involucral bracts. Corollas usually actinomorphic.
Anthers often glandular. Anther appendages hollow or rudimentary. Anther thecae
ecalcarate and ecaudate. Stylar branches usually almost glabrous, with clavate
appendages. Cypsela walls with phytomelan (phytomelanin; smooth or with
spines), usually with twin hairs. Pappus usually uniseriate, consisting of
plumose bristles, scales, or absent. x = 17. Mono-ester pyrrolizidine alkaloids
(secreted by nectaries), pentaynene acetylenes, monoterpenes, sesquiterpene
lactones, ent-kaurine diterpene glycoside, kolavane derivatives, chromenes, and
benzofurans present.
Perityleae B. G.
Baldwin in B. G. Baldwin, B. L. Wessa et J. L. Panero, Syst. Bot. 27: 192. 4
Mar 2002
8/82–87. Galeana (2; G.
hastata, G. pratensis; Central America), Unxia (2;
U. camphorata, U. suffruticosa; Panamá, northern South
America), Villanova (4; V. achilleoides, V.
oppositifolia, V. robusta, V. titicacensis; Mexico to
Chile), Lycapsus (1; L. tenuifolius; Desventuradas Island in
Chile), Amauria (3; A. brandegeana, A. carterae,
A. rotundifolia; southwestern United States, Mexico),
Eutetras (2; E. palmeri, E. pringlei; Mexico),
Pericome (3; P. caudata, P. macrocephala, P.
spilanthoides; southwestern United States, Mexico), Perityle
(65–70; southwestern United States, Mexico). – Western United States,
Mexico, Central America to Chile, Desventuradas Island, with their highest
diversity in western United States and Mexico. Shrubs or herbs. Leaves usually
opposite, glandular. Capitula radiate or discoid, with uni- or biseriate
subequal navicular involucral bracts. Ray corollas often trilobate, disc
corollas quadrilobate (or quinquelobate). Anther thecae ecalcarate and
ecaudate. Style deeply bilobate, branches with short hairs. Cypsela walls with
phytomelan (phytomelanin). Pappus consisting of usually two bristles and crown
of (rudimentary) scales, or absent. n = 19.
 |
Phylogeny of the main clades of Asteraceae based on DNA
sequence data (Funk & al. 2009). Panero & al. (2014) and Mandel
& al. (2017) found Famatinanthus to be sister to all
Asteraceae except
Barnadesioideae.
|
 |
Phylogeny of Asteroideae,
Corymbium and Cichorioideae based on DNA sequence
data showing the tribes (Funk & al. 2009).
|
Richard, Mém. Mus. Natl. Hist. Nat. Paris 6: 74.
Nov 1820 [‘Calycereae’], nom. cons.
Boopidaceae Cass. in
Bull. Sci. Soc. Philom. Paris 1816: 160. 12 Oct 1816
[’Boopideae’]; Boopidales Cass. ex
Bercht. et J. Presl, Přir. Rostlin: 255. Jan-Apr 1820
[’Boopideae’]; Calycerales Link,
Handbuch 1: 815. 4-11 Jul 1829 [‘Calycereae’]
Genera/species 6/47–50
Distribution Southeastern
United States to tropical South America, the Andes to southern South America,
the Falkland Islands.
Fossils Pollen fossils
(Psilatricolporites protrudens) are known from the Miocene of the
Chubut province in Argentina (Palazzesi & al. 2010).
Habit Usually bisexual (rarely
monoecious? or gynomonoecious?, in Acicarpha often andromonoecious
with male flowers in centre), usually perennial (rarely annual) herbs
(occasionally somewhat woody at base).
Vegetative anatomy Phellogen
ab initio superficial? Pericyclic fibres absent. Vascular bundles separate.
Secondary lateral growth normal or absent. Vessel elements usually with simple
(sometimes pseudoscalariform) perforation plates; lateral pits alternate,
bordered pits. Imperforate tracheary xylem elements libriform fibres with
simple pits, non-septate? (also vasicentric tracheids). Secondary wood rays
absent. Axial parenchyma apotracheal diffuse, or paratracheal, vasicentric, or
absent. Phloem rays in Acicarpha surrounded by sclerenchyma. Sieve
tube plastids S type. Nodes 1:1, unilacunar with one leaf trace. Secretory
cavities absent. Primary cortex and medulla with calciumoxalate crystals.
Trichomes Hairs uniseriate or
absent.
Leaves Alternate (spiral),
simple or pinnately compound, entire or pinnately lobed, with ? ptyxis.
Stipules and leaf sheath absent. Petiole vascular bundles? Venation pinnate.
Stomata anomocytic. Cuticular wax crystalloids partly as large glabrous
partially inrolled scales, partly as reticulate to annular threads or small
scales. Secretory cavities absent. Leaf margin serrate or entire.
Inflorescence Terminal,
probably cymose, head-like or capitulum-like pseudanthia (sometimes compound),
surrounded by involucre consisting of one or two whorls of bracts;
inflorescences principally developing centripetally or centrifugally.
Flowers Actinomorphic or
zygomorphic. Epigyny. Sepals (four or) five (or six), small, thick, with open?
aestivation, often aerenchymatous, often modified into spines, persistent,
usually connate. Petals (four or) five (or six), with valvate or open
aestivation, often persistent, connate into campanulate or tubular corolla,
with outer layer separating and photosynthesizing. Nectariferous glands (four
or) five (or six; sometimes absent), alternating with stamens. Disc, uniting
corolla base with style, surrounding corolla tube and expanding into glandular
areoles.
Androecium Stamens (four or)
five (or six), haplostemonous, antesepalous, alternipetalous. Filaments at
least at base connate into tube, adnate to corolla tube (epipetalous), in upper
part usually with ring of thick-walled cells? Anthers connivent to connate at
least at base, basifixed, non-versatile, tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits), sometimes with appendage. Tapetum secretory,
with multinucleate cells. Staminodia absent. Secondary pollen display
present.
Pollen grains
Microsporogenesis simultaneous. Pollen grains tricolporate, with intercolpar
depressions, shed as monads, bicellular at dispersal. Exine tectate, with
columellate infratectum (with furcate columellae), imperforate, spinulate to
granulate or almost smooth.
Gynoecium Pistil composed of
two? (or five?) connate carpels. Ovary inferior, unilocular (pseudomonomerous
by reduction of one locule or perhaps incompletely quinquelocular due to
reduction of septa). Style single, simple, narrow, with secondary pollen
presentation. Stigma punctate to capitate, indistinctly papillate, Dry type;
pollen grains deposited on stigmatic apex and “pumped up” through stylar
elongation. Pistillodium absent.
Ovules Placentation apical.
Ovule one per carpel, anatropous, pendulous, apotropous, unitegmic,
tenuinucellar. Integument more than ten? cell layers thick; outer cell layers
of integument containing chloroplasts. Megagametophyte monosporous,
Polygonum type. Endosperm development cellular. Endosperm haustoria
absent. Embryogenesis solanad.
Fruit An achene with
persistent, often accrescent and sometimes lignified calyx (and often corolla).
Fruit apex with persistent bases of corolla and style. Fruits of marginal
flowers in Acicarpha usually connate.
Seeds Aril absent. Testa
indistinct (undifferentiated). Perisperm not developed. Endosperm copious to
sparse, oily. Embryo straight, well differentiated, chlorophyll? Cotyledons
two. Germination phanerocotylar?
Cytology n = 8
(Acicarpha), 12, 13, 15, 17, 18, 20–22 – Polyploidy frequently
occurring (probably dominating).
DNA Mitochondrial gene
rpl2 absent (lost).
Phytochemistry Insufficiently
known. Flavonols (kaempferol, quercetin), 6-methoxyflavonols, Route I iridoids,
Group VI secoiridoids (e.g. secologanin) and caffeic acid present. Ellagic
acids, tannins, proanthocyanidins and cyanogenic compounds not found.
Carbohydrates stored as oligo- or polyfructosans (e.g. inulin) with isokestose
bonds (starch absent).
Use Unknown.
Systematics
‘Boopis’ (c 13; the Andes, southern Brazil, Argentina;
non-monophyletic), ‘Moschopsis’ (8; Chile, Patagonia in Argentina;
non-monophyletic), Acicarpha (5; A. bonariensis, A.
lanata, A. procumbens, A. runcinata, A.
tribuloides; southeastern United States to tropical South America),
‘Calycera’ (11; Chile, Argentina; non-monophyletic),
‘Gamocarpha’ (6–7; Chile, Argentina; non-monophyletic),
‘Nastanthus’ (4; N. agglomeratus, N.
falklandicus, N. patagonicus, N. spathulatus; the Andes
in Chile and Argentina; non-monophyletic).
Calyceraceae are in most
analyses recovered as sister-group to Asteraceae. All genera except
Acicarpha appear to be non-monophyletic (Denham & al. 2016).
Tank & Donoghue (2010) found the topology
[Boopis+[Moschopsis+Acicarpha]].
 |
Phylogeny (simplified) of
Calyceraceae
based on DNA sequence data (Denham & al. 2016).
|
de Jussieu, Gen. Plant.: 163. 4 Aug 1789, nom.
cons.
Lobeliaceae Juss. ex
A. J. A. Bonpland, Descr. Pl. Malmaison: 19, ad t. 7. Sep 1813, nom. cons.;
Jasionaceae Dumort., Anal. Fam. Plant.: 28, 30. 1829
[’Jasionideae’]; Lobeliales Link,
Handbuch 1: 636. 4-11 Jul 1829 [‘Lobeliaceae’];
Campanulopsida Bartl., Ord. Nat. Plant.: 120, 146.
Sep 1830; Cyphiaceae A. DC. in A. P. de Candolle,
Prodr. 7(2): 497. late Dec 1839; Nemacladaceae Nutt.
in Trans. Amer. Philos. Soc., ser. 2, 8: 254. 15 Dec 1842;
Dortmannaceae Rupr., Fl. Ingr.: 649. Mai 1856
[’Dortmanniaceae’]; Cyananthaceae J.
Agardh, Theoria Syst. Plant.: 384. Apr-Sep 1858 [’Cyanantheae’];
Cyphocarpaceae (Miers) Reveal et Hoogl. in Phytologia
79: 74. 29 Apr 1996
Genera/species
74/2.150–>2.200
Distribution Cosmopolitan
except extreme polar regions; few representatives in Malesia, Australia and New
Zealand.
Fossils Pollen grains of
Campanulaceae are
known from the Oligocene onwards. Fossil seeds often assigned to Campanulaceae as
Campanula palaeopyramidalis have been found in Miocene layers in
Poland.
Habit Usually bisexual (rarely
dioecious or gynodioecious), usually perennial (sometimes annual or biennial)
herbs (sometimes evergreen shrubs, small trees or lianas). Some species are
aquatic. Others are xerophytic.
Vegetative anatomy Phellogen
ab initio superficial or inner cortical. Medullary vascular bundles often
present (cortical bundles rare). Endodermis often prominent. Vascular cylinder
present. Vessel elements usually with simple (sometimes scalariform)
perforation plates; lateral pits scalariform to alternate, simple or bordered
pits? Imperforate tracheary xylem elements libriform fibres with simple or
bordered pits, septate or non-septate? Wood rays uniseriate or multiseriate,
heterocellular (Lobelioideae), usually four to eight cells wide
(Campanuloideae). Axial parenchyma paratracheal scanty vasicentric
(Lobelioideae), or indistinct to absent (Campanuloideae).
Sieve tube plastids S type. Nodes usually 1:1, unilacunar with one leaf trace
(rarely tri- or pentalacunar with three or five? traces). Phloem with
articulated anastomosing laticifers containing white, orange, yellow, dark or
colourless (watery) latex. Cystoliths sometimes present. Calciumoxalate
crystals acicular.
Trichomes Hairs unicellular or
multicellular, usually uniseriate, sometimes stellate (rarely prickles or
dendritic hairs), often calcified/silicified; glandular hairs absent.
Leaves Usually alternate
(spiral; rarely opposite or verticillate), usually simple (rarely pinnately
compound), usually entire (rarely pinnately lobed), with various ptyxis (in
Lobelia supervolute). Stipules absent; leaf base rarely sheathing.
Petiole vascular bundle transection incurved-arcuate. Venation pinnate, usually
craspedodromous or camptodromous (rarely parallelodromous). Stomata usually
anomocytic. Cuticular wax crystalloids partly as large glabrous more or less
inrolled scales, partly as reticulate to annular threads or small scales.
Hydathodes frequent. Leaf margin serrate, crenate or entire, often with
hydathodes.
Inflorescence Usually terminal
(sometimes axillary) panicle, raceme-, spike-, umbel- or headlike cymose
(sometimes pseudanthium with involucre of bracts) particularly in
Campanuloideae, or racemose especially in Lobelioideae
(flowers sometimes solitary axillary, rarely solitary terminal; in
Ruthiella epiphyllous).
Flowers Actinomorphic or
zygomorphic (in Lobelioideae usually resupinate). Usually epigyny
(rarely half epigyny; in Cyananthus hypogyny). Sepals (three to) five
(to ten), with imbricate or valvate aestivation, persistent in fruit, usually
connate; median sepal abaxial. Petals (four or) five (to ten), usually with
valvate (in Cyphocarpus induplicate-valvate) aestivation, connate into
usually campanulate, infundibuliform or tubular corolla (in
Nemacladoideae bilabiate, with upper lip trilobate and lower lip
bilobate; in Cyphocarpus bilabiate, with upper lip entire and lower
lip quadrilobate; in Lobelioideae bilabiate or unilabiate, usually
with upper lip bilobate and lower lip trilobate [in resupinated flower]; in
Cyphia either bilabiate, with upper lip consisting of three and lower
lip of two free petals, or five petals connate into tubular corolla); corolla
tube formation early. Nectariferous disc intrastaminal, annular (in
Adenophora enlarged, gland-like).
Androecium Stamens (three to)
five (to ten), haplostemonous, antesepalous, alternipetalous. Filaments free or
more or less connate, free from tepals or adnate to base of corolla tube
(epipetalous); filament base often widened and enclosing nectariferous disc
(filaments in Nemacladus and Parishella with special
multicellular appendages). Anthers free, often connivent, or connate into tube
surrounding style and stigma, usually basifixed (in Berenice
dorsifixed), non-versatile?, tetrasporangiate, introrse, longicidal (dehiscing
by longitudinal slits). Tapetum secretory, with binucleate cells. Staminodia
absent. Secondary pollen display via combination of, i.a., protandry, anther
tube surrounding style and stigma, anthers dehiscing prior to stylar
elongation, and style with pollen-collecting hairs.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (3–)5–10-colpate,
(2–)3–6-colporate or 3–8-zonoporate (rarely 12–20-pantoporate), usually
shed as monads (in Namacodon as tetrads), usually tricellular
(sometimes bicellular) at dispersal. Exine tectate or semitectate, with
columellate infratectum, imperforate or perforate, spinulate
(Cyphioideae), echinate to verrucate (Campanuloideae,
Nemacladoideae), reticulate-striate (Lobelioideae) or almost
psilate.
Gynoecium Pistil composed of
two, three or five connate carpels. Ovary usually inferior (rarely
semi-inferior or superior), usually bilocular to quinquelocular (sometimes
unilocular; rarely sexalocular to decemlocular by ingrowth of ovary wall).
Style single, simple, usually with hairs on which pollen grains are deposited
(secondary pollen display); stylar hairs capable of invaginating and hence
dislodging pollen grains from anthers. Stigma usually bilobate, trilobate or
quinquelobate, papillate or non-papillate, Dry or Wet type (in
Cyphocarpus with abaxial pollen-collecting hairs; in Cyphia
with liquid-filled porous cavity). Pistillodium absent.
Ovules Placentation usually
axile (rarely apical, basal or parietal [when ovary unilocular]; in
Cyphocarpus free central). Ovules usually numerous per carpel,
anatropous, horizontal, unitegmic, tenuinucellar. Integument approx. six to
eight cell layers thick (sometimes vascularized). Hypostase absent.
Megagametophyte monosporous, Polygonum type. Synergids sometimes with
a filiform apparatus. Endosperm development cellular. Endosperm haustoria
chalazal and micropylar. Embryogenesis solanad. Polyembryony occurring.
Fruit Usually a loculicidal
capsule (sometimes septicidal or poricidal capsule or irregularly dehiscing;
in, i.a., Burmeistera, Canarina, Clermontia a berry;
in, e.g., Craterocapsa, Lysipomia, Parishella
pyxidium; in Theodoravia schizocarp).
Seeds Aril absent. Testa one
to four cell layers thick, sometimes winged. Operculum formed by endosperm
haustorium. Exotesta consisting of cuboidal or fibriform cells with lignified
walls. Only outer epidermis persistent. Endotestal cell walls (especially inner
ones) thickened. Perisperm not developed. Endosperm copious, usually oily
(rarely starchy). Embryo small, straight, without chlorophyll. Cotyledons two.
Germination phanerocotylar.
Cytology n = 9
(Nemacladoideae, Cyphioideae); n = 6–21, 23–30, 32,
34–36, 40, 42, 45, 48, 51, 52 (17 most frequent) (Campanuloideae); n
= 6–14, 19, 21, 35, 70 (Lobelioideae) – Polyploidy and aneuploidy
frequently occurring.
DNA Plastid genome extensively
rearranged, with numerous inversions and losses. Plastid inverted repeat
expanded into small single copy region and sometimes contracted at end of large
single copy region. Deletion of 5 bp in plastid gene ndhF. Plastid
gene infA lost/defunct (Campanula with pseudogene,
Platycodon, Prismatocarpus, Trachelium,
Lobelia). Plastid gene accD, ORF244 and ORF2280 lost in
numerous species of Campanuloideae and Lobelioideae.
Mitochondrial gene rpl2 lost. Mitochondrial intron coxII.i3
lost.
Phytochemistry Flavonols
(quercetin, kaempferol), afzelechin, flavonoid sulphates, biflavonoids,
p-coumaric acid (absent in Lobelioideae), cyanidin,
dammaranes, cantleyoside, oleanolic acid derivatives, ursolic acid,
sesquiterpene lactones, caffeic acid (absent in Lobelioideae) or
chelidonic acid (present in Lobelioideae), chlorogenic acid, toxic
pyrrolizidine and pyridine alkaloids (lobelin etc., in latex of
Lobelioideae), cyanogenic glycosides (linamarin, lotaustralin,
proacacipetalin, prunasin, sambunigrin, triglochinin, zierin), tyrosine derived
cyanogenic compounds, saponins (platycodin, rare, in, e.g.,
Platycodon), fatty acid derived 14-C-polyacetylenes,
aliphatic tetrahydropyrane derivatives, amides, asarone, germacrane-like
compounds, myo-inisitol, chiro-inositols (pinitol,
quebrachitol), lignans (pinoresinol), arbutin, sinapic acid, ferulic acid,
anthraquinones, acetophenones, and polysterols (absent from
Lobelioideae) present. Myricetin, iridoids, ellagic acid, tannins, and
proanthocyanidins not found. Carbohydrates stored as oligo- or polyfructosanes
(e.g. inulin) with isokestose bonds (starch usually absent, although also
present in Cyphia).
Use Ornamental plants,
medicinal plants (Lobelia inflata, Codonopsis pilosula,
etc.), vegetables (Campanula rapunculus, C. rapunculoides,
Centropogon, Platycodon grandiflorus).
Systematics Campanulaceae may be
sister-group to Rousseaceae.
A topology of Campanulaceae may be the
following:
[[Nemacladoideae+Campanuloideae]+[Cyphioideae+Lobelioideae+Cyphocarpus]].
The sister-group relationship of
Cyphocarpus (Cyphocarpoideae) is unresolved and this small
lineage should perhaps be included in Lobelioideae (cf. i.a. Haberle
1998 and Ayers & Haberle 1999).
[Nemacladoideae+Campanuloideae]
Flowers actinomorphic. Pollen grains
spheroidal to oblate-spheroidal, verrucate or beset with spiculae. Style with
retractile hairs in long upper part (Nemacladoideae?). Cell nucleus
with fibrillar protein bodies.
Nemacladoideae (Nutt.)
M. H. G. Gust., Phylogen. Stud. Asterales sensu lato: 34. 1996
2/14. Nemacladus (13;
southwestern United States, northwestern Mexico), Pseudonemacladus (1;
P. oppositifolius; Mexico). – Southwestern United States, Mexico.
Usually annual (sometimes perennial) herbs. Flowers usually zygomorphic, often
resupinate. Hypogyny or half epigyny. Corolla with three adaxial and two
abaxial lobes. Filaments connate into tube, sometimes adnate to corolla lobes
(epipetalous); two dorsal filaments sometimes with groups of small reflexed
structures (pseudonectaries) at base; abaxial fimbriate scales sometimes
present at insertion point of filaments on corolla lobes. Anthers free,
flipping back after pollen release. Pollen grains tricolporate or hexacolpate.
Pistil composed of two (or three) connate carpels. Stylar base with prominent
glands; pollen display by stylar head bending towards or away from median
sepal. Fruit in Parishella a pyxidium. n = 9
Campanuloideae
Burnett, Outlines Bot.: 942, 1094, 1110. Feb 1835
[‘Campanulidae’]
44/900–950. Mainly northern temperate
regions of the Old World (very few in Australia and New Zealand). Usually
perennial (sometimes annual) herbs. Roots often thick. Vessel elements
sometimes with scalariform perforation plates. Polysterol-rich latex present in
laticifers in stem and leaves. Inflorescence usually cymose. Flowers
actinomorphic at anthesis. Median calyx lobe adaxial. Petals in, e.g., Phyteuma
coherent yet open laterally. Staminal bases concealing nectar. Anthers in
Ostrowskia with placentoid. Pollen grains also colpate or porate.
Tectum echinate. Pistil composed of (two or) three to five (to ten) connate
usually antesepalous (rarely antepetalous) carpels (rarely one carpel) or
median carpel adaxial. Ovary in some species of Wahlenbergia
almost superior. Style (at least upper part) covered by long retractile
pollen-collecting and pollen-presenting hairs with bulbous base. Integument in
Ostrowskia massive. Fruit with lateral dehiscence, with pores or
slits. Epicotyl and hypocotyl usually not prolonged. n = 6–21, 23–30, 32,
34–36, 40, 42, 45, 48, 51, 52; x = 6 or more (usually 17). Mesophyll cell
nuclei sometimes with unique type of fibrillar protein inclusions. Large
rearrangements in plastid genome. Plastid DNA in Trachelium
with seven to ten extensive insertions (alternatively four insertions and three
pseudogenes). Intron absent from plastid gene trnI in at least
Campanula garganica. Plastid gene accD, ORF244 and ORF2280
absent in numerous species. Caffeic acid, p-coumaric acid,
polyacetylenes (14-C-aliphatic tetrahydropyran derivates) present.
Cyanantheae Meisn.,
Plant. Vasc. Gen.: Tab. Diagn. 273, Comm. 180. 5-11 Apr 1840
10/64. Platycodon (1; P.
grandiflorus; northeastern Asia), Cyclocodon (4; C.
axillaris, C. lancifolius, C. parviflorus, C.
truncatus; tropical and East Asia), Echinocodon (1; E.
lobophyllus; China), Ostrowskia (1; O. magnifica;
Turkestan), Canarina (3; C. abyssinica: Ethiopia; C.
canariensis: the Canary Islands; C. eminii: tropical East
Africa); Cyananthus (23; the Himalayas); Codonopsis (c 22;
Central and East Asia, Southeast Asia, Malesia), Pankycodon (1; P.
purpureus; the Himalayas, Nepal, Assam, Tibet, western and central China),
Himalacodon (1; H. dicentrifolius; southern Tibet, Nepal,
Sikkim), Pseudocodon (8; the Himalayas, Nepal, northern Burma, Bhutan,
southeastern Tibet, southwestern China). – Leaves often opposite. Stamens
sometimes three. Pollen grains colp(or)ate. Pistil composed of five carpels.
Fruit a loculicidal capsule dehiscing by valves, or a berry. n = 7–9 (17).
Platycodin (saponin) present in, i.a., Platycodon. – According to
Hong & Wang (2015), Cyananthus is sister to the clade
[Codonopsis+[Pankycodon+[Himalacodon+Pseudocodon]]],
and the clade
[Platycodon+[[Echinocodon+Cyclocodon]+[Canarina+Ostrowskia]]
is sister-group to the remaining Cyanantheae
(=Platycodonoideae sensu Hong & Wang).
[Wahlenbergieae+Campanuleae]
Wahlenbergieae
Endl., Gen. Plant.: 514. Jun 1838
16/340–350. Siphocodon (2; S.
debilis, S. spartioides; Western Cape), Rhigiophyllum
(1; R. squarrosum; Western Cape), Feeria (1; F.
angustifolia; Morocco), ’Wahlenbergia’ (260–270;
warm-temperate to tropical regions on both hemispheres, with their highest
diversity on the Southern Hemisphere; polyphyletic), Kericodon (1;
K. crispus; Western and Eastern Cape), Nesocodon (1; N.
mauritianus; Mauritius), Heterochaenia (3; H. borbonica,
H. ensifolia, H. rivalsii; Réunion), Berenice (1;
B. arguta; Réunion), Microcodon (3; M. glomeratus,
M. hispidulus, M. linearis; Northern and Western Cape),
Craterocapsa (5; C. alfredica, C. congesta, C.
insizwae, C. montana, C. tarsodes; South Africa,
Swaziland, Lesotho, Zimbabwe), Namacodon (1; N. schinzianum;
central Namibia), Gunillaea (2; G. emirnensis, G.
rhodesica; tropical Africa, Madagascar), ’Prismatocarpus’ (c
30; tropical and southern Africa, with their highest diversity in the Cape
Provinces; paraphyletic; incl. Merciera?, Roella?),
Roella (c 20; Western Cape, one species in Eastern Cape and
KwaZulu-Natal; in Prismatocarpus?), Merciera (6; M.
azurea, M. brevifolia,
M. eckloniana, M. leptoloba, M. tenuifolia, M.
tetraloba; Western Cape; in Prismatocarpus?), Treichelia
(2; T. dodii, T. longibracteata; Western Cape). –
Warm-temperate to tropical regions on both hemispheres, with their highest
diversity in southern Africa. Filaments adnate in lower part to petals
(epipetalous). Pollen grains porate. Fruit a loculicidal capsule dehiscing by
apical valves, pores or opercula. Hypocotyl and epicotyl usually not elongated.
n = 6 or more. – The Western Cape clade
[Siphocodon+Rhigiophyllum] is sister-group to the remaining
Wahlenbergieae, according to Cupido & al. (2013), but
Feeria was not included in their analyses. At least the majority of
the other genera are nested inside ‘Wahlenbergia’.
Campanuleae Dumort.,
Fl. Belg.: 58. 1827
18/590–>610. Jasione (13–16;
Europe, the Mediterranean, southwestern Asia), Musschia (3; M.
aurea, M. wollastonii: Madeira; M. isambertoi: Islas
Desertas), ’Campanula’ (500–>520; temperate regions on the
Northern Hemisphere, tropical mountains; non-monophyletic), Legousia
(7; L. falcata, L. hybrida, L. julianii, L.
pentagonia, L. scabra, L. skvortsovii, L.
speculum-veneris; Europe, the Mediterranean; in Campanula?),
Triodanis (7; T. biflora, T. coloradoensis, T.
holzingeri, T. lamprosperma, T. leptocarpa, T.
perfoliata, T. texana; southern Canada, United States, one
species, T. perfoliata, also in Central and South America to
Argentina; in Campanula?), Petromarula (1; P.
pinnata; Crete; in Campanula?), Phyteuma (c 40; Europe,
the Mediterranean, temperate Asia; in Campanula?),
Physoplexis (1; P. comosa; the Alps; in
Phyteuma/Campanula?), Trachelium (2; T.
caeruleum, T. lanceolatum; Europe, the Mediterranean),
Homocodon (2; H. brevipes, H. pedicellatum; Bhutan,
southwestern China; in Campanula?), Favratia (1; F.
zoysii; the Alps), Zeugandra (2; Z. iranica, Z.
iranshahrii; Iran), Peracarpa (1; P. carnosa; tropical
Asia; in Campanula?), Heterocodon (1; H. rariflorum;
southwestern Canada, western United States; in Campanula?),
Githopsis (4; G. diffusa, G. pulchella, G.
specularioides, G. tenella; Vancouver Island, western United
States, California, Baja California; in Campanula?),
Cryptocodon (1; C. monocephalus; Pamir),
Cylindrocarpa (1; C. sewerzowii; Kyrgyzstan), Sergia
(2; S. regelii: Tadzhikistan; S. sewerzowii: Kazakhstan). –
Temperate regions on the Northern Hemisphere, tropical mountains. Filaments
adnate in lower part to petals (epipetalous). Pollen grains porate, triangular
in polar view. Fruit a poricidal or valvicidal capsule laterally dehiscing (due
to activity of axicorn on drying; Shulkina & al. 2003), or dry indehiscent.
Hypocotyl and epicotyl usually not elongated. n = 6 or more. – Most genera in
Campanuleae are nested inside Campanula (Borsch & al.
2009; Haberle & al. 2009; Zhou & al. 2012).
[Lobelioideae+Cyphioideae+Cyphocarpoideae]
Flowers zygomorphic. Pollen grains
prolate. Stylar apex present at base of newly opened anthers; hairs restricted
to stylar apex.
Lobelioideae Burnett,
Outlines Bot.: 942, 1094, 1110. Feb 1835 [‘Lobelidae’] (under
construction)
26/1.180–>1.200. ‘Lobelia’
((>420; nearly cosmopolitan; paraphyletic), Grammatotheca (1;
G. bergiana; Western and Eastern Cape, KwaZulu-Natal; in Lobelia?),
Dialypetalum (5; D. compactum, D. floribundum,
D. humbertianum, D. montanum, D. stenopetalum;
Madagascar), Wimmerella (10; southern Africa), Dielsantha (1;
D. galeopsoides; Central Africa), Monopsis
(13; tropical and southern Africa), Isotoma
(12; Australia; in Lobelia?), Ruthiella (4; R.
oblongifolia, R. saxicola, R. schlechteri, R.
subcordata; New Guinea), Diastatea (6; D. costaricensis,
D. expansa, D. ghiesbreghtii, D. micrantha, D.
tenera, D. virgata; central and southern Mexico, Central America,
one species, D. micrantha, also in the Andes to northern Argentina),
Solenopsis (7; S. antiphonitis, S. balearica, S.
bicolor, S. bivonae, S. corsica, S. laurentia,
S. minuta; the Canary Islands, the Mediterranean to Turkey, Cyprus and
Syria), Downingia
(13; southwestern Canada, western United States, one species, D.
pusilla, also in Chile), Porterella (1; P. carnosula;
western North America), Legenere (1–2; L. limosa, L.
valdiviana; California, Chile), Howellia (1; H.
aquatilis; western United States), Lysipomia (c 30; the high
Andes), Burmeistera (c 115; Guatemala to Peru, with the highest
diversity in the Andes of Colombia and Ecuador), ‘Centropogon’ (c
220; tropical America, especially in the Andes;
non-monophyletic),‘Siphocampylus’ (c 220; tropical America,
especially in the Andes; non-monophyletic), Hippobroma (1; H.
longiflora; the West Indies; in Lobelia?),
Heterotoma (1; H. lobelioides; Mexico, Central America),
Sclerotheca (10; Rarotonga, the Society Islands, Marquesas Islands,
Rapa Island), Trematolobelia
(4; T. grandifolia, T. kauaiensis, T. macrostachys,
T. singularis; the Hawaiian Islands; in Lobelia?), Brighamia
(2; B. insignis, B. rockii; the Hawaiian Islands; in
Lobelia?), Delissea
(9; the Hawaiian Islands; in Lobelia?), ‘Cyanea’
(c 60; the Hawaiian Islands; non-monophyletic; in Lobelia?), Clermontia
(22; the Hawaiian Islands; in Lobelia?). – Subcosmopolitan, although
largely tropical and with their highest diversity in Central America and
tropical South America. Annual or perennial herbs, small (sometimes pachycaul)
trees. Leaves with supervolute ptyxis (Lobelia).
Flowers split zygomorphic, with upper and lower lips separate down to petal
base; usually with upper lip bilobate and lower lip trilobate, or upper lip
absent and lower lip quinquelobate (sometimes with upper lip trilobate and
lower lip bilobate). Flowers usually resupinate by torsion of pedicel (Lobelia
and closely allied; flower appearing to have ’normal’ orientation due to
resupination, with median corolla lobe abaxial). Filaments connate at least
apically. Anthers usually connate into tube surrounding style (sometimes free).
Pollen grains usually reticulate, striate. Pistil composed of two connate
carpels. Extrafloral nectaries occasionally present on ovary. “Pollen pump”
mechanism often present. Style pushing up from inside anther tube and exposing
pollen grains; style with brush hairs and pollen in pollen box. Stigma Wet
type. Synergids hooked. Antipodal cells usually persistent. Fruit rarely a
berry or a pyxidium; or a capsule dehiscing laterally. n = 6–14, 19, 21, 35,
70; x = 6 or more (usually 7 and 14). Plastid DNA in Lobelia
with three large deletions including parts of clpP. Plastid DNA with
two inversions in at least 25 species of Lobelia
and in Sclerotheca jayorum, five inversions in Lobelia erinus
and L. fervens, three inversions in Lobelia cardinalis,
L. holstii and Monopsis lutea. Plastid gene clpP
absent (lost) in at least Lobelia holstii and Monopsis lutea.
Plastid gene accD, ORF244 and ORF2280 absent in numerous species.
Pyridine and pyrrolizidine alkaloids (in latex), and chelidonic acid present.
Caffeic acid, p-coumaric acid and polysterols not found. – A large
part of the Lobelioideae genera are nested inside Lobelia
(Antonelli 2008). Gigantism has developed in Lobelia in at least four
areas: the East African tropical mountains, the Andes, the Hawaiian Islands,
and the Himalayas (Chen & al. 2016).
Cyphioideae Walp. in
Ann. Bot. Syst. 2: 1037. 12-15 Mai 1852 [‘Cyphiaceae’]
1/c 65. Cyphia
(c 65; Africa, the Cape Verde Islands, with their largest diversity in southern
Africa). – Perennial herbs (often twining) with tuberous root. Flowers
zygomorphic, sometimes almost actinomorphic. Corolla with upper lip bilobate
and lower lip trilobate, or upper lip trilobate and lower lip bilobate, or
upper lip absent and lower lip quinquelobate. Filaments connate at base.
Anthers sometimes slightly connivent. Pollen grains psilate. Pistil composed of
two carpels. Ovary semi-inferior. Style bending away from median sepal, not
elongating after anther dehiscence; stylar hairs with bulbous base (bristle
hairs absent); pollen grains deposited in “pollen box”. Stigma with
fluid-filled terminal cavity with usually lateral (sometimes terminal) pore.
Fruit a septicidal and loculical capsule (valves bifid). Endosperm also with
starch. n = 9.
Cyphocarpoideae Miers
in London J. Bot. 7: 61. 1848 [‘Cyphocarpaceae’]
1/3. Cyphocarpus (3; C.
innocuus, C. psammophilus, C. rigescens; Chile). –
Perennial or annual herbs. Leaves deeply lobed. Flowers zygomorphic. Epigyny.
Corolla with induplicate-valvate aestivation, with upper (adaxial) lip
unilobate, cucullate, with apical appendage, and lower lip quadrilobate.
Filaments free from each other, adnate in lower part to corolla lobes
(epipetalous). Pistil composed of two carpels. Ovary strongly elongated. Fruit
laterally dehiscent. n = 9. Cell nuclei with fibrillar inclusions. –
Cyphocarpus should possibly be included in Lobelioideae.
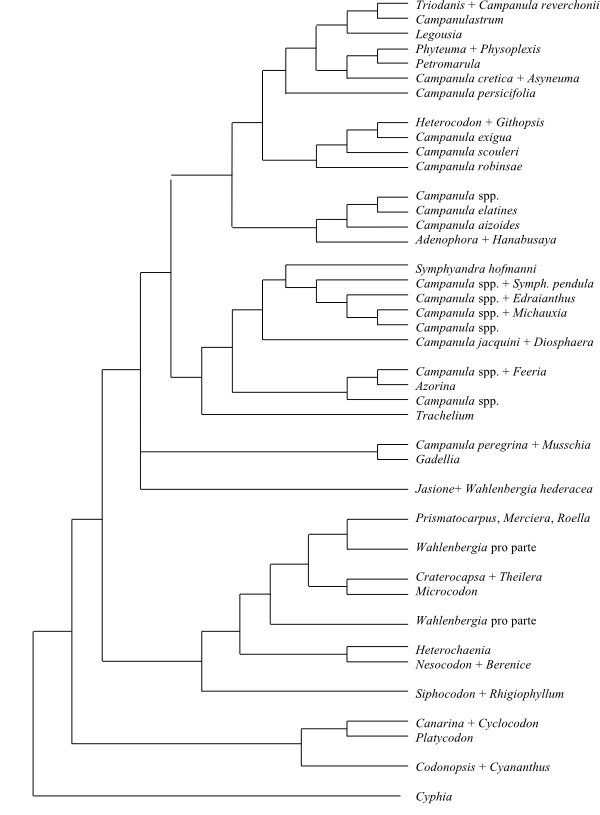 |
Bayesian majority rule consensus tree of
Campanuloideae and Cyphia
based on DNA sequence data (Haberle & al. 2009).
Cyclocodon is sister-group to Canarina
according to Borsch & al. (2009). Similar results were obtained by
Hong & Wang (2015), although Wahlenbergia hederacea was
sister to the remaining Wahlenbergieae.
|
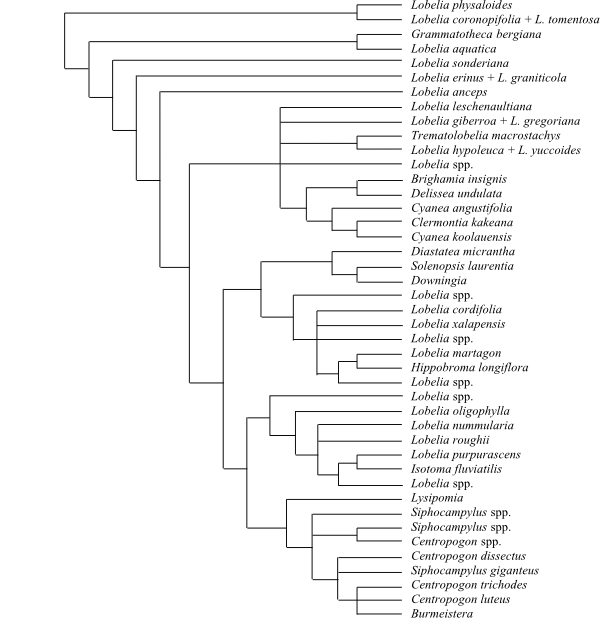 |
Bayesian inference tree (simplified) of
Lobelioideae based on DNA sequence data (Antonelli 2008).
|
Brown, Prodr. Fl. Nov.-Holl.: 573. 27 Mar 1810
[’Goodenoviae’], nom. cons.
Goodeniales R. Br. ex
Bercht. et J. Presl, Přir. Rostlin: 252. Jan-Apr 1820
[‘Goodenoviae’]; Brunoniaceae Dumort.,
Anal. Fam. Plant.: 19, 21. 1829, nom. cons.;
Scaevolaceae Lindl., Intr. Nat. Syst. Bot.: 189. 27
Sep 1830 [‘Scaevoleae’]; Brunoniales
Lindl., Nix. Plant.: 20. 17 Sep 1833; Scaevolales R.
Br. in C. F. P. von Martius, Consp. Regn. Veg.: 32. Sep-Oct 1835
[‘Scaevoleae’]
Genera/species 11/435–450
Distribution Tropical and
subtropical coastal areas along the Atlantic, the Indian and the Pacific
Oceans, Australia, Tasmania, islands in the Pacific and the Indian Oceans, with
their largest diversity in Western Australia and Tasmania.
Fossils Pollen
(Poluspissusites) from Oligocene and later strata are reported from
Cameroon, New Zealand, Australia and Patagonia.
Habit Bisexual perennial or
annual herbs, suffrutices or shrubs (rarely climbing or arborescent). Many
representatives are xerophytic.
Vegetative anatomy Phellogen
ab initio subepidermal or cortical. Medullary vascular bundles often present.
Secondary lateral growth normal or absent. Vessel elements usually with simple
(sometimes scalariform) perforation plates, lateral pits usually alternate
(sometimes scalariform or intermediate), usually bordered (in Scaevola
sometimes simple) pits. Imperforate tracheary xylem elements tracheids and
fibre tracheids with bordered pits, non-septate. Wood rays uniseriate or
multiseriate, homocellular or heterocellular, or absent. Axial parenchyma
apotracheal diffuse, and/or paratracheal scanty vasicentric, or absent. Sieve
tube plastids S type. Nodes usually 1:1, unilacunar with one leaf trace
(sometimes 3:3, trilacunar with three traces, or 5:5, pentalacunar with five
traces). Laticifers absent. Heartwood with gum-like substances. Idioblasts with
branched sclereids present especially in foliar mesophyll. Prismatic
calciumoxalate crystals or druses present in parenchyma cells in many
species.
Trichomes Hairs simple,
dendritic or stellate, often verrucose/strigose, sometimes candelabra-shaped,
or absent; in Brunonia with short thin-walled basal cell and
bicellular or multicellular arm inserted in right angle at basal cell and
pressed to surface; glandular hairs with multicellular stalk (sometimes
peltate-lepidote).
Leaves Usually alternate
(spiral; in Scaevola section Enantophyllum opposite), simple,
entire, often coriaceous, with ? ptyxis. Stipules and leaf sheath absent.
Petiole vascular bundles? Venation pinnate. Stomata usually anomocytic (in
Brunonia paracytic with four subsidiary cells, two of which parallel
to guard cells). Cuticular wax crystalloids? Mesophyll often with
sclerenchymatous idioblasts containing branched sclereids. Leaves often with
axillary hair tuft. Leaf margin serrate or entire (rarely sinuate).
Inflorescence Terminal or
axillary, racemose or thyrsoid, raceme-, spike- or umbel-like (in
Brunonia spicate to capitular, terminal; flowers rarely solitary
axillary). Inflorescences sometimes head-like pseudanthia with involucre of
bracts.
Flowers Zygomorphic (in
Brunonia almost actinomorphic). Usually epigyny or half epigyny (in
Brunonia and some species of Goodenia hypogyny). Sepals
usually small, usually five (rarely three, sometimes indistinct), with open to
imbricate aestivation, often persistent, connate. Petals five, with
(induplicate-)valvate aestivation, unilabiate with five lower lobes, or
bilabiate with two upper and three lower lobes (rarely with spur), connate,
with odd lobe ventral, and usually with wings along margins (except in
Brunonia and Selliera) each corolla lobe appearing trilobate.
Nectary usually absent; one or two intrastaminal nectariferous glands sometimes
present. Disc present or absent.
Androecium Stamens five,
haplostemonous, antesepalous, alternipetalous. Filaments free from each other,
usually adnate at base to corolla tube (epipetalous; in some species of
Goodenia free from tepals). Anthers basifixed, non-versatile?,
tetrasporangiate, introrse, longicidal (dehiscing in bud by longitudinal
slits), connivent or connate and forming tube around style. Tapetum secretory,
with binucleate to multinucleate cells. Staminodia absent. Secondary pollen
display present.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (in
Leschenaultia 4–8-por[or]ate), usually shed as monads (in
Leschenaultia as rhomboidal tetrads), bicellular at dispersal. Exine
tectate, with columellate infratectum (with furcate columellae), imperforate to
finely perforate, rugulate (Dampiera, Leschenaultia) or
microspinulate.
Gynoecium Pistil composed of
usually four (in Brunonia two) connate carpels; usually only two
carpels fertile. Ovary usually inferior or semi-inferior (in Brunonia
and some species of Goodenia superior), bilocular at base, unilocular
at centre, and bilocular above (sometimes entirely unilocular; in species of
Scaevola quadrilocular; in Brunonia unilocular with septum at
base only). Style single, usually simple, narrow, curved, with pollen-catching
and often hairy indusium (enclosing pollen initially; indusium glabrous in,
i.a., Brunonia) and collar- or cup-shaped outgrowth at apex
immediately below stigma (style in some species of Goodenia bifid to
quadrifid, each branch with indusium); style “pumping up” pollen grains
during its elongation (pollen in Brunonia caught by stylar hairs).
Stigma truncate or bilobate, papillate, Dry type. Pistillodium absent.
Ovules Placentation
basal-axile (in Brunonia basal) or parietal (when ovules situated in
centre of ovary). Ovules usually several or numerous per carpel (in
Brunonia, and some species of Dampiera, Goodenia and
Scaevola one per fertile carpel), usually anatropous (rarely
campylotropous), usually ascending, unitegmic, tenuinucellar. Integument six to
20 cell layers thick (in Levenhookia four). Hypostase present in at
least some species (absent in Brunonia). Megagametophyte monosporous,
Polygonum type, with multicellular chalazal process. Synergids
elongate, hooked. Antipodal cells sometimes persistent. Endosperm development
cellular (in Dampiera rarely nuclear?). Endosperm haustoria absent.
Embryogenesis solanad.
Fruit Usually a septicidal
(and sometimes loculicidal) laterally dehiscing capsule (sometimes a drupe or
achene with persistent calyx, rarely a schizocarp with one-seeded nutlike
mericarps).
Seeds Aril usually absent
(present in Coopernookia?). Testa sometimes winged, seven to 14 cell
layers thick (in Brunonia thin, compressed). Exotestal cells usually
palisade, with thickened walls (especially inner walls; not in
Brunonia), often containing crystals. Both outer and inner epidermis
persistent. Endotesta developed into endothelium? Hypodermal layers sometimes
lignified. Perisperm not developed. Endosperm usually copious (in
Brunonia thin or absent), oily. Embryo well developed, straight,
chlorophyll? Cotyledons two (in Brunonia swollen). Germination
phanerocotylar.
Cytology n = 16, 18, 24, 27,
32, 36, 45; x = 7–9 – Polyploidy frequently occurring.
DNA Mitochondrial gene
rpl2 possibly lost. rpl16 intron lost. Plastid gene
infA lost/defunct (Goodenia). Intron lost in plastid gene
rpoC1 in Goodenia and Scaevola.
Phytochemistry
O-methyl flavonols, Route I iridoids, Group VI secoiridioids (e.g.,
secologanin; Brunonia lacks iridoids), ursolic acid, caffeic acid,
chlorogenic acid, alkaloids, saponins, cyanogenic compounds, and polyacetylenes
present. Flavonols, ellagic acid and proanthocyanidins not found. Carbohydrates
stored as oligo- or polyfructosans (e.g. inulin in roots and rhizome) with
isokestose bonds.
Use Ornamental plants.
Systematics Leschenaultia
(26–27; southern New Guinea, Australia, with their largest diversity in
southwestern Western Australia), Anthotium (4; A. humile,
A. junciforme, A. odontophyllum, A. rubriflorum;
southwestern Western Australia), Dampiera
(c 65; Australia, with their highest diversity in southwestern Western
Australia); Brunonia (1; B. australis; Australia, Tasmania);
Scaevola
(120–130; Socotra, Australia, Tasmania, Melanesia, New Zealand, Polynesia to
the Hawaiian Islands, Cuba, some species also along coasts of tropical
Southeast Asia, Malesia, tropical America, Africa, Madagascar, the Mascarene
Islands, southern India and Sri Lanka), Coopernookia (6; C.
barbata, C. chisholmii, C. georgei, C.
polygalacea, C. scabridiuscula, C. strophiolata;
southwestern Western Australia, southern South Australia, eastern New South
Wales), ‘Goodenia’
(185–190; New Guinea, Australia, one species also in Malesia to southern
China, G. konigsbergeri on Java; non-monophyletic), Selliera
(1; S. radicans; southeastern South Australia, Victoria, southeastern
New South Wales, Tasmania, New Zealand, Stewart Island, temperate South
America), Velleia (21; New Guinea, Australia, Tasmania),
Verreauxia (5; V. dyeri, V. paniculata, V.
reinwardtii, V. verreauxii, V. villosa; southwestern
Western Australia), Pentaptilon (1; P. careyi; western
Western Australia). – Scaevola collaris, Selliera radicans,
Velleia and Verreauxia are nested inside Goodenia
(Gardner & al. 2016).
Goodeniaceae are sister-group
to [Calyceraceae+Asteraceae], although Soltis
& al. (2007) identified Goodeniaceae as sister to
Calyceraceae.
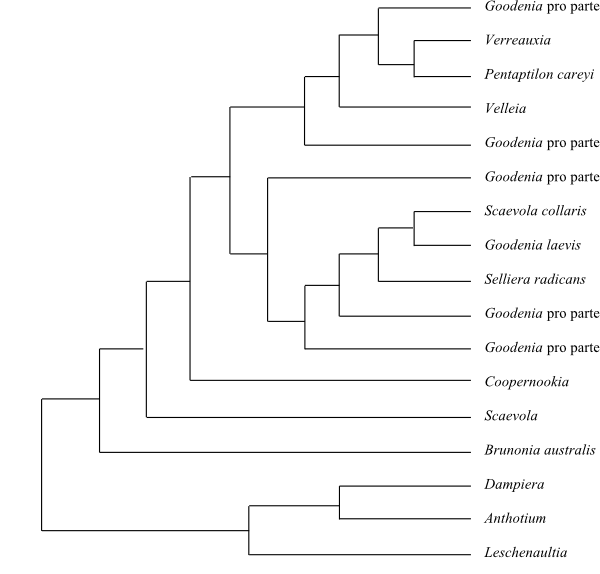 |
Cladogram of Goodeniaceae (50%
majority-rule from a partitioned Bayesian inference analysis) based on
DNA data (Jabaily & al. 2012). A similar topology was recovered by
Gardner & al. (2016).
|
Dumortier, Anal. Fam. Plant.: 20, 25. 1829
[’Menyanthideae’], nom. cons.
Menyanthales Bercht.
et J. Presl, Přir. Rostlin 1(115): 1. 1825 [‘Menyantheae’]
Genera/species 6/60–65
Distribution Cosmopolitan
except polar areas, with their largest diversity in Australia.
Fossils A Menyanthes
seed was reported from the Miocene of Poland. Fossil pollen
(Striasyncolpites laxus) from the Oligocene and Miocene of New
Zealand, Central Europe and Patagonia is assigned to Menyanthaceae.
Habit Usually bisexual (rarely
monoecious, dioecious or gynodioecious), usually perennial (rarely annual)
herbs. Hydrophytes (aquatic) or helophytes.
Vegetative anatomy Phellogen?
Vascular tissue atactostele-like, with separate and often scattered stem
bundles. Cortical vascular bundles present. Cortex and mesophyll with
intercellular canals and spaces. Secondary lateral growth normal or absent.
Vessel elements usually with simple (rarely scalariform) perforation plates;
lateral pits alternate? Imperforate tracheary xylem elements ? Wood rays
absent. Axial parenchyma absent. Sieve tube plastids S type. Endodermis
sometimes prominent. Nodes 3:3, trilacunar with three leaf traces, or 5:5,
pentalacunar with five traces. Parenchyma in some species with idioblasts
containing asterosclereids and projecting into intercellular spaces.
Crystals?
Trichomes Hairs absent.
Leaves Alternate (spiral or
distichous), usually simple (in Menyanthes trifoliolate), entire, in
Nymphoides sometimes anisomorphous, with involute ptyxis. Stipules
absent; petiole sometimes sheathing and decurrent. Colleters present. Petiole
with lateral wings. Petiole vascular bundle transection arcuate to annular.
Leaf base in Nephrophyllidium wide. Venation palmate or pinnate.
Stomata anomocytic, often present at adaxial side only. Cuticular wax
crystalloids? Mesophyll with or without sclerenchymatous idioblasts containing
asterosclereids. Leaf margin usually entire (sometimes slightly serrate or
crenate), usually glanduliferous, often with hydathodes.
Inflorescence Terminal
panicle, raceme, umbel or capitate, or flowers paired or solitary axillary.
Flowers Actinomorphic, often
large. Hypogyny (probably secondary) or half epigyny. Sepals usually five
(rarely four), persistent, usually connate in lower part. Petals usually five
(rarely four), usually with valvate (rarely weakly imbricate) aestivation,
connate in lower part, with margins or adaxial surface often fimbriate or hairy
and with wings (broad edges). Lateral petal traces fused, possibly indicating
primary zygomorphy. Nectariferous disc intrastaminal, usually surrounding ovary
base. Heterostyly frequent.
Androecium Stamens usually
five (rarely four), haplostemonous, antesepalous, alternipetalous. Filaments
free from each other, adnate at base to corolla tube (epipetalous). Anthers
dorsifixed or basifixed, versatile, tetrasporangiate, introrse, longicidal
(dehiscing by longitudinal slits). Tapetum secretory, with multinucleate cells
(nuclei fused). Female flowers with staminodia; fimbriate scale-like staminodia
sometimes alternating with stamens.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually 3(–6)-colp(or)ate
(Menyanthes, Nephrophyllidium) or parasyncolpate
(Liparophyllum, Nymphoides, Villarsia), shed as
monads, usually tricellular (sometimes bicellular) at dispersal. Exine tectate,
with columellate infratectum, imperforate?, striate to rugulate
(Menyanthes, Nephrophyllidium), or striate, rugulate or
smooth (Liparophyllum, Nymphoides, Villarsia).
Gynoecium Pistil composed of
two connate carpels. Ovary superior (probably secondarily) or semi-inferior,
unilocular, with nectariferous glands at base. Style single, bifid, or absent.
Stigmas lobate or spatulate, papillate, Wet type. Male flowers with
pistillodium.
Ovules Placentation (often
intrusively) parietal. Ovules numerous per carpel, anatropous, horizontal,
unitegmic, tenuinucellar. Integument more than ten? cell layers thick.
Hypostase present or absent. Megagametophyte monosporous, Polygonum
type. Endosperm development cellular. Endosperm haustoria absent. Embryogenesis
asterad.
Fruit Usually a septicidal
and/or loculicidal or irregularly dehiscing capsule (in Nymphoides
sometimes nutlike; in Liparophyllum a berry).
Seeds Aril absent. Seed coat
exotestal. Testa sometimes winged, sometims with air-filled and/or hooked
hairs. Elaiosome (caruncle rich in lipids) present in some species. Exotestal
cells with thickened outer walls, often with various surface processes.
Mesotesta and endotesta usually crushed (sometimes with sclerotized cells).
Perisperm not developed. Endosperm copious, oily. Embryo well developed,
straight, chlorophyll? Cotyledons two. Germination?
Cytology x = 9
(Menyanthes, Nymphoides, Villarsia); x = 17
(Nephrophyllidium); n = 8, 9, 18, 20, 27, 28, 54 – Polyploidy
occurring. Protein bodies present in nucleus.
DNA Intron absent from plastid
gene rpl2 (entire gene absent?).
Phytochemistry Flavonols
(kaempferol, quercetin etc.), Group VI secoiridoids (e.g. secologanin), Group
VII secoiridoids (e.g. sweroside), Group X secoiridoids (e.g. loganin), caffeic
acid, iridoid alkaloids, and saponins present. Ellagic acid, tannins,
proanthocyanidins and cyanogenic compounds not found. Carbohydrates stored as
oligo- or polyfructosans (e.g. inulin in roots and rhizome) with isokestose
bonds (starch absent).
Use Ornamental plants,
medicinal plants.
Systematics Menyanthes
(1; M. trifoliata; temperate regions on the Northern Hemisphere),
Nephrophyllidium (1; N. crista-galli; northern Japan,
northwestern North America); Ornduffia
(6; O. albiflora, O. calthifolia, O. marchantii,
O. parnassifolia, O. reniformis, O. submersa;
southwestern Western Australia, South Australia, eastern Queensland to
Victoria, Tasmania), Villarsia
(3; V. capensis, V. goldblattiana, V. manningiana;
Western Cape), Liparophyllum
(8; southwestern Western Australia, South Australia, eastern Queensland to
Victoria, Tasmania, New Zealand), Nymphoides
(c 40; nearly cosmopolitan).
Menyanthaceae are sister to
the clade [Goodeniaceae+[Calyceraceae+Asteraceae]].
[Menyanthes+Nephrophyllidium]
is sister-group to the remainder, whereas Ornduffia
is sister to [Villarsia+[Nymphoides+Liparophyllum]].
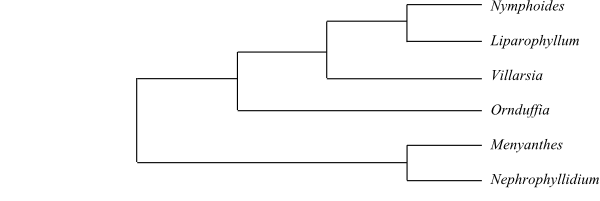 |
Cladogram of Menyanthaceae based
on DNA sequence data and morphology (Tippery & Les 2009; Tippery
& al. 2009).
|
Agardh, Theoria Syst. Plant.: 95. Apr-Sep 1858
[’Pentaphragmeae’], nom. cons.
Pentaphragmatales
Doweld, Tent. Syst. Plant. Vasc.: liv. 23 Dec 2001
Genera/species 1/25–30
Distribution Southeast Asia,
southern China, Malesia to New Guinea, with their largest diversity in West
Malesia.
Fossils Unknown.
Habit Usually bisexual (rarely
dioecious), perennial herbs, large and often somewhat succulent. Rooting at
stem base.
Vegetative anatomy Phellogen
absent. Endodermis conspicuous. Vessel elements with scalariform perforation
plates; lateral pits scalariform. Imperforate tracheary xylem elements fibre
tracheids with bordered pits, often septate. Wood rays uniseriate, biseriate,
homocellular, or absent. Axial parenchyma absent. Wood not storied. Sieve tube
plastids S type? Nodes unilacunar? with ? leaf traces. Laticifers and latex
probably absent. Crystals absent.
Trichomes Hairs unicellular
and uniseriate, or multicellular and uniseriate or branched. Foliar hairs often
multicellular and branched, often with uniseriate branches.
Leaves Alternate (distichous),
simple, entire, usually with strongly asymmetrical base, with ? ptyxis.
Stipules and leaf sheath absent. Petiole vascular bundles? Venation pinnate.
Stomata anomocytic or anisocytic, surrounded by three or four cells slightly
different from normal epidermal cells. Cuticular wax crystalloids? Leaf margins
usually serrate (sometimes entire).
Inflorescence Axillary,
cymose, usually scorpioid (helicoid). Bracts usually conspicuous,
membranous.
Flowers Actinomorphic or
slightly zygomorphic. Hypanthium usually present, adnate to ovary by five
longitudinal septa creating five nectariferous pits. Epigyny. Sepals two large
and three small, with imbricate aestivation, petaloid, persistent, often
connate. Petals (four or) five, with valvate aestivation, usually fleshy or
cartilaginous, persistent, usually connate at base, with marginal wings. Five
nectar-producing antepetalous pores, lacunae, inserted between five
longitudinal septa fusing hypanthium with ovary. Disc absent.
Androecium Stamens (four or)
five, haplostemonous, antesepalous, alternipetalous. Filaments flat, free from
each other, in sympetalous species adnate to corolla tube (epipetalous).
Anthers basifixed, non-versatile, tetrasporangiate, extrorse, longicidal
(dehiscing by longitudinal septa). Tapetum secretory, with binucleate cells.
Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains (2–)3-colporate or
(2–)3-colpate, trilobate and pleurotreme in polar view, with apertures
between lobes, shed as monads, bicellular at dispersal. Exine tectate, with
columellate infratectum, imperforate?, smooth; endexine lamellate.
Gynoecium Pistil composed of
two (or three) connate carpels. Ovary inferior, usually bilocular (rarely
trilocular), inserted at hypanthium by (four or) five narrow longitudinal
septa, being continuations of staminal filaments and forming (four or) five
nectariferous canals or pits. Style single, simple, short and thick. Stigma
capitate or clavate, type? Pistillodium absent.
Ovules Placentation axile
(placentae bipartite). Ovules numerous per carpel, anatropous, pendulous,
unitegmic, tenuinucellar. Integument approx. three cell layers thick. Hypostase
absent. Megagametophyte monosporous, Polygonum type, protruding from
micropyle (extramicropylar). Endosperm development cellular. Endosperm
haustorium micropylar, unicellular and uninucleate (chalazal endosperm
haustorium absent). Embryogenesis solanad.
Fruit A many-seeded berry with
persistent perianth.
Seeds Seeds minute. Aril
absent. Exotestal cells cuboidal, with lignified inner walls. Endotesta
developing into an endothelium? Perisperm not developed. Endosperm copious,
starchy. Suspensor short. Embryo straight, well developed, two thirds the
length of the seed, chlorophyll? Cotyledons two. Germination?
Cytology n = 54–56
DNA Mitochondrial gene
rpl2 absent (lost).
Phytochemistry Very
insufficiently known. Inulin and alkaloids not found. Iridoids?
Use Vegetables.
Systematics
Pentaphragma (25–30; southern Burma, southern China, Indochina,
Malesia to New Guinea, with their largest diversity in West Malesia).
Pentaphragma may be sister to the
remaining Campanulales
above the [Rousseaceae+Campanulaceae] clade,
although the support is sometimes low.
Takhtajan, Sist. Filogen. Cvetk. Rast. [Syst.
Phylog. Magnolioph.]: 343, 374. 4 Feb 1967 [’Phellineaceae’]
Phellinales Doweld,
Tent. Syst. Plant. Vasc.: liv. 23 Dec 2001;
Phellinanae Doweld, Tent. Syst. Plant. Vasc.: liv. 23
Dec 2001
Genera/species 1/10
Distribution New Caledonia.
Fossils Fossil leaf fragments
of Phelline has been reported from the Early Miocene of New Zealand
(Pole 2010).
Habit Dioecious, evergreen
trees or shrubs.
Vegetative anatomy Phellogen?
Vessel elements with scalariform perforation plates (end walls almost
vertical); lateral pits scalariform or opposite (in one row), bordered pits.
Imperforate tracheary xylem elements tracheids or fibre tracheids with bordered
pits, non-septate. Wood rays uniseriate or multiseriate (up to 14 cells wide
and often more than 2 cm tall), heterocellular. Axial parenchyma apotracheal
diffuse, or paratracheal scanty. Sieve tube plastids S type. Nodes 3?:3?,
trilacunar? with three? leaf traces. Acicular calciumoxalate crystals,
styloids, crystal sand and other crystal types present.
Trichomes Hairs uniseriate,
often reddish-brown, tufted.
Leaves Alternate (spiral),
simple, entire, often coriaceous, with ? ptyxis. Stipules and leaf sheath
absent. Axillary hairs present. Petiole vascular bundle transection annular.
Venation pinnate. Stomata anomocytic, with very large guard cells, with inner
and outer ledges. Cuticular wax crystalloids as platelets and rodlets.
Sclerenchymatic tissue enclosing veins. Leaf margin crenate-sinuate or
entire.
Inflorescence Axillary spike-
or raceme-like or paniculate cymose.
Flowers Actinomorphic, small.
Hypogyny. Sepals four or five (or six), with more or less open aestivation,
minute, persistent, connate at base. Petals four or five (or six), with valvate
aestivation, fleshy, with small incurved apex, free. Nectary absent. Disc
absent.
Androecium Stamens four or
five (or six), haplostemonous, antesepalous, alternipetalous. Filaments free
from each other and from tepals. Anthers basi-dorsifixed, non-versatile,
tetrasporangiate, introrse, longicidal (dehiscing by longitudinal slits).
Tapetum glandular? Female flowers with staminodia.
Pollen grains
Microsporogenesis simultaneous? Pollen grains tricolpor(oid)ate, shed as
monads, ?-cellular at dispersal. Exine tectate, with columellate infratectum,
perforate, microechinulate or microrugulate.
Gynoecium Pistil composed of
(two or) four or five (or six) connate carpels. Ovary superior, usually
quadrilocular or quinquelocular (sometimes bilocular or sexalocular). Style
single, simple, very short or absent. Stigma usually quadrilobate or
quinquelobate, type? Male flowers with pistillodium.
Ovules Placentation axile to
apical. Ovule one per carpel, somewhat campylotropous to hemitropous,
pendulous, apotropous, unitegmic, tenuinucellar. Integument ? cell layers
thick. Megagametophyte monosporous, Polygonum type? Endosperm
development? Endosperm haustoria? Embryogenesis?
Fruit A drupe with one to five
separate pyrenes.
Seeds Aril absent. Exotesta
present. Endotesta? Perisperm not developed. Endosperm copious. Embryo small,
chlorophyll? Cotyledons two. Germination?
Cytology n = 17
DNA Mitochondrial gene
rpl2 absent?
Phytochemistry Insufficiently
known. Benzylisoquinoline alkaloids (homoerythrine alkaloids: homoerythrine,
homoerythroidine, homoazaerythrine, holidine, etc.) present. Route I iridoids?
Carbohydrates stored as oligo- or polyfructosans (e.g. inulin) with isokestose
bonds (starch absent).
Use Unknown.
de Candolle, Prodr. 7(2): 521. late Dec 1839
[‘Roussaeaceae’]
Carpodetaceae Fenzl
in Denkschr. Königl.-Baier. Bot. Ges. Regensburg 3: 155. 28 Nov 1841
[’Carpodeteae’]; Abrophyllaceae Nakai,
Chosakuronbun Mokuroku [Ord. Fam. Trib. Nov.]: 243. 20 Jul 1943;
Rousseales Doweld, Tent. Syst. Plant. Vasc.: liii. 23
Dec 2001
Genera/species 4/14
Distribution Mauritius, New
Guinea, Solomon Islands, Vanuatu, eastern Australia, New Zealand incl. Stewart
Island.
Fossils Unknown.
Habit Usually bisexual (in
Carpodetus sometimes gynodioecious), evergreen trees or shrubs
(Roussea often climbing).
Vegetative anatomy Phellogen?
Young stem with separate vascular bundles. Vessel elements with scalariform
perforation plates; lateral pits scalariform, opposite or alternate, bordered
pits. Imperforate tracheary xylem elements tracheids or fibre tracheids with
bordered pits, non-septate. Wood rays uniseriate or multiseriate,
heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates,
or paratracheal scanty (Roussea). Sieve tube plastids S type. Nodes
3:3, trilacunar with three leaf traces. Pericycle and leaf mesophyll in
Abrophyllum and Cuttsia with latex idioblasts consisting of
vertically elongate cells arranged in longitudinal rows and filled with white
brittle content, and ring of sclerenchyma. Parenchyma cells in
Carpodetus with rhomboidal crystals.
Trichomes Hairs unicellular,
usually curved, thick-walled, verrucose; glandular hairs in
Abrophyllum and Cuttsia with short sunken stalk and
unicellular head; hairs in Roussea unicellular conical or
glandular.
Leaves Usually alternate
(spiral; in Roussea opposite), simple, entire, with ? ptyxis. Stipules
and leaf sheath absent. Petiole vascular bundle transection arcuate or annular.
Venation usually semicraspedodromous (in Roussea craspedodromous).
Stomata anomocytic (Roussea, Carpodetus), in
Abrophyllum and Cuttsia with two almost circular cells.
Cuticular wax crystalloids? Petiole with radially elongate schizogenous
resinous canals (Roussea). Mesophyll in Carpodetus with
crystalliferous idioblasts. Petiole and abaxial side of lamina with
multicellular peltate glandular hairs (Roussea); domatia as pockets
sometimes present in Carpodetus. Leaf margin more or less glandular
serrate.
Inflorescence Usually terminal
or axillary panicle (flowers in Roussea few or solitary, axillary).
Flowers Actinomorphic, usually
small (in Roussea large). Usually hypogyny (in Carpodetus
epigyny to half epigyny). Sepals (four or) five (to seven), with valvate
aestivation, unequally long, persistent (Roussea) or caducous, free or
connate at base. Petals (four or) five (to seven), with valvate aestivation,
thick, persistent (Roussea) or caducous, usually free (in
Roussea connate in lower part). Nectariferous disc intrastaminal, in
Roussea quadrilobate or quinquelobate, or absent.
Androecium Stamens (four or)
five (to seven), haplostemonous, antesepalous, alternipetalous. Filaments free
from each other and from tepals, in Abrophyllum and Cuttsia
inserted at margin of nectariferous disc, in Roussea inserted on lobes
of nectariferous disc. Anthers usually basifixed (in Cuttsia
dorsifixed?), non-versatile, tetrasporangiate, usually introrse (in
Roussea extrorse), longicidal (dehiscing by longitudinal slits).
Tapetum secretory, with binucleate cells. Female flowers with staminodia.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (in
Roussea penta- or hexapantoporate, or penta- or
hexapantobrachycolpate), usually shed as monads (in Carpodetus as
tetrads), usually bicellular (in Carpodetus tricellular) at dispersal.
Endoapertures in Cuttsia H-shaped. Exine tectate, with columellate
infratectum, striate to rugulate (Abrophyllum, Cuttsia), or
smooth to somewhat uneven (Carpodetus, Roussea).
Gynoecium Pistil composed of
(three to) five (to seven) antepetalous carpels. Ovary usually superior
(sometimes inferior or semi-inferior), usually quinquelocular (sometimes tri-,
quadri-, sexa- or septalocular; Roussea). Style single, usually simple
(in Roussea thick, persistent; in Carpodetus narrow; in
Cuttsia apically branched) or absent. Stigma capitate
(Carpodetus, Roussea), or (trilobate to) quinquelobate, type?
Male flowers with pistillodium.
Ovules Placentation axile.
Ovules numerous per carpel, anatropous, unitegmic, tenuinucellar. Integument
five to eight cell layers thick. Megagametophyte monosporous,
Polygonum type. Suspensor filiform (Roussea). Endosperm
development ab initio nuclear. Endosperm haustorium micropylar
(Roussea). Embryogenesis?
Fruit Usually a berry, in
Roussea sharply quinqueangular (to septangular), in
Abrophyllum and Cuttsia with persistent stigma, in
Carpodetus (trilocular to) quinquelocular and leathery (in
Cuttsia a loculicidal capsule).
Seeds Aril absent. Anticlinal
and inner periclinal walls of exotestal cells more or less massively thickened
(in Carpodetus entirely). Mesotesta and endotesta crushed
(Roussea). Perisperm not developed. Endosperm copious, with aleuron
and lipids. Embryo small, straight, chlorophyll? Cotyledons two.
Germination?
Cytology n = 14, 15
(Carpodetus)
DNA Mitochondrial gene
rpl2 absent?
Phytochemistry Insufficiently
known. Flavonols (kaempferol, quercetin), triterpenes (lupeol), and
prodelphinidins present (Carpodetus). Tannins absent in
Roussea.
Use Unknown.
Systematics Rousseaceae are probably
sister-group to Campanulaceae.
Rousseoideae Horan.,
Char. Ess. Fam.: 110. 17 Jun 1847 [‘Roussaeariae’]
1/1. Roussea (1; R.
simplex; Mauritius). – Evergreen small tree or liana. Resiniferous
canals present. Hairs tufted-stellate; glandular hairs peltate. Leaves opposite
or verticillate. Petiole vascular bundle transection annular (with
endodermis?). Leaf margins serrate. Bud scales present. Flowers terminal,
solitary. Flowers usually pentamerous (sometimes tetramerous). Hypogyny. Calyx
large, with valvate aestivation. Petals large, connate at base. Anthers
basifixed, extrorse, entirely fused with straight connective. Pollen grains
hexaporate or octaporate. Pollen embedded in slime when liberated. Tectum
almost imperforate (complete). Pistil composed of five to seven connate
carpels. Style apically expanding, persistent, gradually widening into ovary.
Stigmatic lobes narrower, erect. Endosperm haustorium micropylar. Fruit a
berry, with persistent calyx with reflexed lobes. Seeds carunculate? Exotestal
cell walls thickened. Mesotesta and endotesta crushed. Embryo relatively long.
n = ? Phytochemistry virtually unknown.
Carpodetoideae (Fenzl)
J. Lundb. in Bot. J. Linn. Soc. 137: 276. 3 Dec 2001
3/13. Carpodetus
(11; New Guinea, Solomon Islands, Vanuatu, New Zealand incl. Stewart Island),
Abrophyllum (1; A. ornans; eastern Queensland, eastern New
South Wales), Cuttsia (1; C. viburnea; southeastern
Queensland, northeastern New South Wales). – New Guinea, eastern Australia,
Melanesia, New Zealand. Evergreen trees. Hairs unicellular, thick-walled,
strongly curved, verrucose. Petiole vascular bundle transection arcuate or
annular; petiole with additional accessory bundles. Inflorescence terminal
panicle. Flowers tetramerous to hexamerous. Calyx caducous. Petals finally
free. Filaments free from each other and from petals. Pollen grains in Carpodetus
shed in tetrads. Pistil composed of three to six connate carpels. Style usually
short (occasionally absent). Stigma capitate (in Cuttsia divided).
Fruit dry, baccate, or a loculicidal and septicidal capsule. Seeds numerous.
Funicle elongate. Anticlinal and inner periclinal exotestal cell walls strongly
thickened (in Carpodetus
all walls thickened). Endosperm with hemicellulose (Carpodetus).
Mesophyll in Abrophyllum and Cuttsia containing white groups
of small unlignified cells, similar to raphide bundles. n = 14, 15. – Carpodetus
is sister to [Abrophyllum+Cuttsia] (Kårehed & al.
1999).
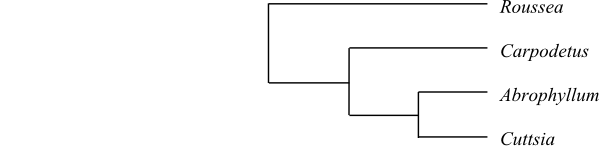 |
Cladogram of Rousseaceae based on
morphology and DNA sequence data (Kårehed & al. 1999).
|
Brown, Prodr. Fl. Nov.-Holl.: 565. 27 Mar 1810
[’Stylideae’], nom. cons.
Stylidiales R. Br. ex
Bercht. et J. Presl, Přir. Rostlin: 253. Jan-Apr 1820
[‘Stylideae’]; Candolleaceae F. Muell.,
Syst. Census Austral. Plant.: 85. late 1882-early 1883, nom. illeg.;
Donatiaceae (Engl.) B. Chandler in Notes Roy. Bot.
Gard. Edinburgh 5: 44. Nov 1911, nom. cons.
Genera/species 4//>320
Distribution: South and
Southeast Asia, mainly extratropical regions of Australia, Tasmania, New
Zealand incl. Stewart Island, southernmost South America.
Fossils Fossil pollen grains,
Tricolpites stylidioides, resembling those in Forstera, have
been reported from Oligocene layers in southeastern Australia.
Habit Usually bisexual
(sometimes monoecious or polygamomonoecious), usually perennial herbs
(sometimes dwarf shrubs; in Donatia and Phyllachne
cushion-shaped). Some species are helophytes and numerous species are
xerophytes.
Vegetative anatomy Phellogen
ab initio cortical. Young stem with separate vascular bundles. Secondary
lateral growth anomalous (via cylindrical cambium, produced outside vascular
bundles, with cambial activitiy mainly unifacial producing tissue towards inner
side). Vessel elements usually with simple (in Donatia scalariform)
perforation plates; lateral pits alternate, bordered pits. Imperforate
tracheary xylem elements fibre tracheids with bordered pits, non-septate. Wood
rays absent. Axial parenchyma absent (Stylidium). Wood elements
partially storied. Interxylary phloem present. Sieve tube plastids S type.
Nodes 1:1, unilacunar with one leaf trace. Crystals?
Trichomes Hairs in
Donatia unicellular, uniseriate, in leaf axils; glandular hairs with
stalk and unicellular or multicellular head (some hairs mucilaginous), or
absent.
Leaves Alternate (spiral),
simple, entire, usually linear, often coriaceous, with ? ptyxis. Stipules and
leaf sheath absent. Petiole absent. Venation usually parallelodromous, often
flabellate or striate (in Donatia acrodromous). Stomata usually
anomocytic (in Donatia magellanica paracytic). Cuticular wax
crystalloids? Mesophyll with idioblasts as mucilage cells, without
sclerenchymatous idioblasts. Leaf margin entire. Leaf apex sometimes with
extrafloral nectary.
Inflorescence Terminal, cymose
(often raceme- or spike-like) or raceme, spike etc., or flowers solitary
terminal (in, i.a., Donatia).
Flowers Zygomorphic and
resupinate (in Levenhookia, Stylidium and most species of
Forstera), or actinomorphic (in Donatia,
Oreostylidium, Phyllachne and some species of
Forstera). Epigyny. Sepals (two to) five (to seven), usually with
imbricate (in Donatia open?) aestivation, persistent, usually connate.
Petals usually five, with imbricate aestivation, unequally sized; odd corolla
lobe laid down ab initio ventrally and gradually reduced (in, e.g.,
Stylidium), or becoming large dome-shaped labellum (in, e.g.,
Levenhookia), although flowers usually resupinating (flowers
semi-resupinate, split-monosymmetric) labellum thus becoming lateral, remaining
four corolla lobes uniform or non-uniform (petals in Donatia five to
ten, equally sized), usually connate and tubular (in Donatia free);
corolla tube formation early; corolla tube mouth often with row of glandular
appendages. Nectary epigynous or absent. Disc extrastaminal, usually bifid
(sometimes unilateral; in Donatia discoid).
Androecium Stamen usually two
(in Donatia two or three), haplostemonous, alternisepalous,
antepetalous. Filaments usually connate and tubular (in Donatia free),
free from tepals, usually completely adnate to style into gynostemium (stamens
in Donatia free from style). Anthers basifixed? (in Donatia
dorsifixed), non-versatile, tetrasporangiate, extrorse, longicidal (dehiscing
by longitudinal slits); thecae arranged head-to-head. Tapetum secretory, with
binucleate cells. Staminodia absent. Secondary pollen display as
androphore-like structure, gynostemium, present as stylar column with stigma
and anthers at apex.
Pollen grains
Microsporogenesis simultaneous. Pollen grains 2–8-colpate (in
Donatia 3–4-colp[or]ate), shed as monads, bicellular or tricellular
at dispersal (in Donatia?). Exine tectate, with usually columellate
infratectum, imperforate to finely perforate, usually spinulate (in
Donatia smooth).
Gynoecium Pistil composed of
usually two (in Donatia two or three) connate carpels. Ovary inferior,
usually bilocular below, unilocular above and sometimes bilocular distally
(sometimes pseudomonomerous due to reduced posterior carpel, or unilocular due
to absence of septum; in Donatia bilocular or trilocular). Stylodia
usually two, usually connate and adnate to filaments (in Donatia two
or three, subulate, free). Stigma bilobate (sometimes bristle-like) or stigmas
capitate, papillate, Dry type. Pistillodium absent.
Ovules Placentation usually
free central in unilocular part of ovary (sometimes free basal; in
Donatia axile in bilocular part of ovary). Ovules usually numerous per
carpel (one or four when one locule fertile), anatropous, pendulous, horizontal
or ascending, unitegmic, tenuinucellar. Integument four to six cell layers
thick. Hypostase present in Donatia. Megagametophyte monosporous,
Polygonum type. Synergids sometimes with a filiform apparatus.
Antipodal cells persistent. Endosperm development cellular. Endosperm haustoria
chalazal and micropylar (micropylar haustorium most aggressive). Embryogenesis
solanad.
Fruit Usually a septicidal
capsule (in, e.g., Donatia a nutlike indehiscent capsule).
Seeds Aril? Testa one to four
cell layers thick. Exotesta? Only outer epidermis persistent. Endotesta
developing into an endothelium? Perisperm not developed. Endosperm copious,
fleshy, oily. Embryo small, well differentiated, chlorophyll? Cotyledons
usually two (rarely one). Germination phanerocotylar.
Cytology n = 24
(Donatia); n = 5–16, 18, (24), 26, 28, 30 (mainly in
Stylidium) – Protein bodies present in nucleus
(Stylidioideae).
DNA Mitochondrial gene
rpl2 absent (lost).
Phytochemistry Flavonols
(kaempferol, quercetin), Route I iridoids (Route I carbocyclic iridoid
resembling monotropein; in Donatia?), caffeic acid, tannins (in
Donatia), proanthocyanidins, and saponins (rare) present. Ellagic
acid, alkaloids, and cyanogenic compounds not found. Secoiridoids?
Carbohydrates stored as oligo- or polyfructosans (e.g. inulin) with isokestose
bonds (starch absent).
Use Ornamental plants.
Systematics Stylidiaceae are sister-group
to the clade [Phellinaceae+[Argophyllaceae+Alseuosmiaceae], according
to Tank & Donoghue (2010). On the other hand, they have sometimes been
recovered as sister to the clade [Menyanthaceae+[Goodeniaceae+[Calyceraceae+Asteraceae]]] or as members of a
trichotomy also comprising these two clades.
Donatioideae Mildbr.
in H. G. A. Engler, Pflanzenr. 35: 18. 26 Mai 1908
1/2. Donatia (2; D.
novae-zelandiae: New Zealand incl. Stewart Island, Tasmania; D.
fascicularis: southernmost Chile and Argentina). – Cushion-shaped dwarf
shrubs. Often xerophytes. Phellogen cortical? Older stem with very thick cortex
and with vascular tissue as a central narrow cylinder. Mucilage cells present.
Hairs uniseriate, axillary. Leaves often ericoid. Venation acrodromous. Stomata
usually paracytic. Flowers solitary, terminal. Flowers actinomorphic. Sepals
three to seven, free. Petals five to ten, free. Stamens two or three. Filaments
free from each other and from petals. Anthers extrorse. Pistil composed of two
or three connate carpels. Stylidia free, recurved. Stigmas capitate. Hypostaste
present. Fruit indehiscent. Suspensor short. n = 24. Tannins present.
Stylidioideae Kitt. in
A. Richard, Nouv. Elém. Bot., ed. 3, Germ. transl.: 827. 1840
[‘Stylideae’]
3//>320. Levenhookia
(10; southwestern Western Australia, southeastern South Australia, Victoria,
New South Wales), Stylidium
(probably more than 300; Bengal, Sri Lanka, Burma, Vietnam, southern coast of
China, the Malay Peninsula, the Philippines, Australia, Tasmania, New Zealand
incl. Stewart Island, with almost all species confined to Australia),
Forstera (9; Tasmania, New Zealand incl. Stewart Island, Auckland
Islands, Campbell Island, one species, F. uliginosa, in southernmost
South America). – Distribution as for Stylidiaceae. Herbs (sometimes
climbing or twining; sometimes cushion-shaped). Phellogen superficial or
outer-cortical. Phelloderm developing centrifugally. Secondary lateral growth
anomalous (secondary tissue developing exclusively towards inside). Cambium
storied, developing from beneath endodermis. Vascular bundles closed, scattered
or annular. Intraxylary phloem present. Xylem (sometimes also phloem)
developing centripetally. Vessel elements with simple perforation plates. Hairs
also axillary. Glandular hairs abundant. Leaves pseudoverticillate or in
rosette (rosette often present at distance above ground). Flowers usually
zygomorphic (in Stylidium
occasionally obliquely zygomorphic; rarely actinomorphic). Sepals connate;
median sepal abaxial, yet often semi-resupinate. Petals usually 2+2
(occasionally five; in Levenhookia
four plus sensitive labellum), connate into tube, often with glandular
appendages forming corona, sometimes with spur. Nectaries also as paired
glands. Stamens two, entirely adnate to style, thus forming gynostemium
(column). Adaxial staminal pair fertile. Anthers inserted near stigma. Thecae
with connivent tips. Entire complex usually sensitive (not in Levenhookia?)
and moving when touched by pollinator (cf. ‘trigger plants’). Pollen grains
2–8-colpate, bicellular or tricellular at dispersal. Pistil composed of two
connate carpels (adaxial carpel strongly reduced). Stigma small, Dry type.
Synergids elongate. Fruit usually a septicidal capsule (sometimes indehiscent).
Seed coat exo-endotestal. Exotestal cell walls sclerotized. Suspensor much
longer than wide. Cotyledon often single. n = 5–16, 18, (24), 26, 28, 30 –
Two monophyletic groups were identified by Wagstaff & Wege (2002). The
first one consists of Forstera.
The second lineage comprises Levenhookia
as sister to Stylidium.
According to Darnowski & al. (2006), there are signs (protease activity in
leaves) indicating that at least a few species may be carnivorous or at least
insect-trapping.
The gynostemium consists, at the
bending point, of an epidermis together with anterior and posterior
parenchymatic layers, the cells of which containing large numbers of starch
grains, mitochondria and vacuoles. The cell walls are rich in pectin and their
cellulose fibrils are almost exclusively circumferential. When the gynostemium
is ready to be “fired”, the anterior parenchyma layer has a high turgor –
balanced by stretched cells with longitudinal cellulose fibrils in the
posterior parenchyma layer – and large KCl concentration. When the sensitive
gynostemium is stimulated by the pollinator, the cell walls at the bending
point will recurve extremely fast transversely and thus “fire” the
gynostemium. This movement takes only about ten to 30 milliseconds –
incrediby fast for a plant.
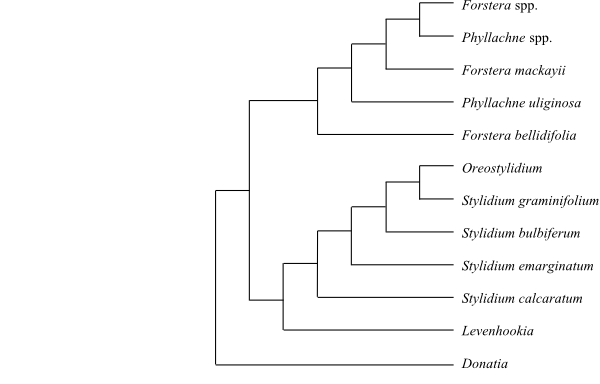 |
Cladogram of Stylidiaceae based on
DNA sequence data (Wagstaff & Wege 2002).
|
Literature
Abid R, Qaiser M. 2002. Genus Inula
L. (s. str.) (Compositae-Inuleae) in Pakistan and Kashmir. – Candollea 56:
315-325.
Abreu VHR de, Conceição Santos J da,
Esteves RL, Gonçalves-Esteves V. 2015. Pollen morphology of Praxelis
(Asteraceae, Eupatorieae,
Praxelinae) in Brazil. – Plant Syst. Evol. 301: 599-608.
Adams LG. 1979. A review of the genus
Solenogyne (Asteraceae)
in Australia and New Zealand. – Brunonia 2: 43-65.
Adamson RS. 1952. A revision of the genera
Prismatocarpus and Roella. – J. South Afr. Bot. 17:
93-166.
Adamson RS. 1954. The genus Merciera
A. DC. – J. South Afr. Bot. 20: 157-163.
Adamson RS. 1955a. The South African species
of Lightfootia. – J. South Afr. Bot. 21: 115-218.
Adamson RS. 1955b. The phytogeography of
Roella and Prismatocarpus. – Svensk Bot. Tidskr. 49:
24-28.
Adegbite AE, Ayodele MS. 2004. Cytogenetic
and phylogenetic studies in the genus Vernonia Schreb. – Feddes
Repert. 115: 513-518.
Adesogan EK, Okunade AL. 1979. A new flavone
from Ageratum conyzoides. – Phytochemistry 18: 1863-1864.
Afzelius K. 1924. Embryologische und
zytologische Studien in Senecio und verwandten Gattungen. – Acta
Horti Berg. 8: 123-219.
Afzelius K. 1949. On chromosome numbers in
Senecio and some allied genera. – Acta Horti Berg. 15: 65-77.
Afzelius K. 1966. Some species of
Pseudogynoxys from Ecuador. – Bot. Not. 119: 233-242.
Afzelius K. 1967. Chromosome numbers in some
Senecioneae. – Svensk Bot. Tidskr. 61: 1-9.
Agababian M. 1997. Centaurea subg.
Centaurea (Compositae): delimitation and distribution of sections and
subsections. – Lagascalia 19: 889-902.
Agnew ADQ. 1961. The genus Picris in
Iraq. – Bull. Coll. Sci. Univ. Baghdad 6: 61-70.
Ahlstrand L. 1978. Embryology of
Ursinia (Compositae). – Bot. Not. 131: 487-496.
Ahlstrand L. 1979a. Embryology of
Arctot[id]eae-Arctoti[di]nae (Compositae). – Bot. Not. 132: 109-116.
Ahlstrand L. 1979b. Embryology of
Arctotideae-Gorteriinae (Compositae). – Bot. Not. 132: 371-376.
Ahlstrand L. 1979c. Embryology of
Arctotideae-Gundeliinae (Compositae). – Bot. Not. 132: 377-380.
Ahlstrand L. 1979d. Embryology of Arctotideae
and Calenduleae (Compositae). – Ph.D. diss., University of Gothenburg,
Sweden.
Ahlstrand L. 1985. Embryology of Calenduleae
(Compositae). – Nord. J. Bot. 5: 79-97.
Ahlstrand L. 1992. Contributions to the
embryology of Arctotideae (Compositae). The genera Dymondia Compton,
Cullumia R. Br., Didelta L’Herit. and Heterolepis
Cass. – Comp. Newslett 22: 1-4.
Airy Shaw HK. 1941 [1942]. Additions to the
flora of Borneo and other Malay islands XIX. The Pentaphragmataceae of the
Oxford University expedition to Sarawak, 1932. – Kew Bull. 1941: 233-236.
Airy Shaw HK. 1954. Pentaphragmataceae. – In:
Steenis CGGJ van (ed), Flora Malesiana I, 4(5), Noordhoff-Kolff N. V., Batavia,
pp. 517-528.
Aksoy N, Duman H, Efe A. 2008. Centaurea
yaltirikii sp. nov. (Asteraceae, C. sect.
Pseudoseridia) from Turkey. – Nord. J. Bot. 26: 53-56.
Aladsesanmi AJ, Snyder JK, Kelley CJ,
Hoffmann JJ. 1991. Homoerythrina alkaloids of Phelline comosa. –
Phytochemistry 30: 3497-3498.
Alarcon R, Ocampos S, Pacciaroni A, Colloca
C, Sosa V. 2009. Constituents of Gutierrezia mandonii (Asteraceae). – Biochem. Syst. Ecol.
37: 683-685.
Alavi SA. 1976. Genus Coleostephus
Cassini in Europe (Asteraceae).
– Phyton (Horn) 17: 319-328.
Aldridge AE. 1975. Taxonomic and anatomical
studies in Sonchus L. subg. Dendrosonchus Webb ex Schultz
Bip. and related genera. – Ph.D. diss., University of Reading, United
Kingdom.
Aldridge AE. 1976. A critical reappraisal of
the Macaronesian Sonchus subgenus Dendrosonchus s.l.
(Compositae-Lactuceae). – Bot. Macaronésica 2: 25-57.
Aldridge AE. 1977. Anatomy and evolution in
the Macaronesian Sonchus subg. Dendrosonchus
(Compositae-Lactuceae). Nodal and petiolar vascular patterns. – Bot.
Macaronésica 3: 41-59.
Aldridge AE. 1978. Anatomy and evolution in
the Macaronesian Sonchus subg. Dendrosonchus
(Compositae-Lactuceae). – Bot. J. Linn. Soc. 76: 249-285.
Aldridge AE. 1979. Evolution within a single
genus: Sonchus in Macaronesia. – In: Bramwell D (ed), Plants and
islands, Academic Press, London, pp. 279-291.
Alexander JCR. 1979. The Mediterranean
species of Senecio sections Senecio and
Delphinifolius. – Notes Roy. Bot. Gard. Edinb. 37: 387-428.
Alford MH. 2012. New records of
Gamochaeta (Asteraceae)
in the Hawaiian Archipelago. – In: Evenhuis NL, Eldredge LG (eds), Records of
the Hawaii Biological Survey for 2011, Bishop Mus. Occas. Pap. 113: 1-6..
Allen GA. 1984. Morphological and cytological
variation in the western North American Aster occidentalis complex (Asteraceae). – Syst. Bot. 9:
175-191.
Al-Shammary KI, Gornall RJ. 1994. Trichome
anatomy of the Saxifragaceae
s.l. from the southern hemisphere. – Bot. J. Linn. Soc. 114: 99-131.
Alvarenga SAV, Ferreira MJP, Rodrigues GV,
Emerenciano VP. 2005. A general survey and some taxonomic implications of
diterpenes in the Asteraceae. –
Bot. J. Linn. Soc. 147: 291-308.
Álvarez Fernández I, Fuertes Aquilar J,
Panero JL, Nieto Feliner G. 2001. A phylogenetic analysis of Doronicum
(Asteraceae, Senecioneae) based on
morphological, nuclear ribosomal (ITS), and chloroplast (trnL-F)
evidence. – Mol. Phylogen. Evol. 20: 41-64.
Amano M, Ohba H. 2000. Chromosome numbers of
some alpine species of Saussurea (Asteraceae) in Nepal Himalaya. – J.
Jap. Bot. 75: 178-184.
Amaro JM, Adrian M. 1982. Diterpenoides del
Oxylobus glanduliferus. – Rev. Latinoamer. Quim. 13: 110-113.
Amin A. 1957. Taxonomic studies in the genus
Launaea. – M.Sc. thesis, Faculty of Science, Cairo University,
Egypt.
Amin A. 1978. A taxonomic revision of the
genus Launaea (Compositae) I. General considerations. – Taeckholmia
9: 111-117.
Ammal EKJ, Sobti SN. 1962. Chromosome
relationships in Calendula species. – Proc. Indian Acad. Sci., Sect.
B, 55: 128-130.
Anderberg AA. 1982. The genus
Anvillea (Compositae). – Nord. J. Bot. 2: 297-305.
Anderberg AA. 1985a. The genus
Iphionopsis (Compositae-Inuleae). – Nord. J. Bot. 5: 51-56.
Anderberg AA. 1985b. The genus
Iphiona (Compositae-Inuleae). – Nord. J. Bot. 5: 169-194.
Anderberg AA. 1986. The genus
Pegolettia (Compositae, Inuleae). – Cladistics 2: 158-186.
Anderberg AA. 1988. The genus
Anisothrix (Compositae-Inuleae). – Bot. Jahrb. Syst. 109:
363-372.
Anderberg AA. 1989. Phylogeny and
reclassification of the tribe Inuleae (Asteraceae). – Can. J. Bot. 67:
2277-2296.
Anderberg AA. 1990. Nablonium is a
congener of Ammobium (Asteraceae-Gnaphalieae). – Telopea
4: 129-135.
Anderberg AA. 1991a. Taxonomy and phylogeny
of the tribe Inuleae (Asteraceae).
– Plant Syst. Evol. 176: 75-123.
Anderberg AA. 1991b. Taxonomy and phylogeny
of the tribe Plucheeae (Asteraceae). – Plant Syst. Evol.
176: 145-177.
Anderberg AA. 1991c. Taxonomy and phylogeny
of the tribe Gnaphalieae (Asteraceae). – Opera Bot. 104:
1-195.
Anderberg AA. 1992a. Cladistics of the
Gnaphalieae, additional data. – Compositae Newsl. 20-21: 35.
Anderberg AA. 1992b. In defence of the
transfer of Nabonium to Ammobium (Asteraceae-Gnaphalieae), a reply to
Orchard. – Telopea 5: 13-19.
Anderberg AA. 1993. Cytology of
Ighermia Wikl. (Asteraceae-Inuleae) with notes on its
systematic position. – Compositae Newsl. 23: 7-9.
Anderberg AA. 1995. Doellia, an
overlooked genus in the Asteraceae-Plucheeae. – Willdenowia
25: 19-24.
Anderberg AA. 2009a. Inuleae. – In: Funk
VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and
biogeography of Compositae, International Association for Plant Taxonomists,
Wien, pp. 667-680.
Anderberg AA. 2009b. Athroismeae. – In:
Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and
biogeography of Compositae, International Association for Plant Taxonomists,
Wien, pp. 681-688.
Anderberg AA, Bremer K. 1991. Parsimony
analysis and cladistic reclassification of the Relhania generic group
(Asteraceae-Gnaphalieae). – Ann.
Missouri Bot. Gard. 78: 1061-1072.
Anderberg AA, Freire SE. 1990a.
Jalcophila boliviensis A. Anderb. & S. Freire, a new species of
South American Asteraceae. –
Brittonia 42: 138-141.
Anderberg AA, Freire SE. 1990b.
Luciliopsis perpusilla Wedd. is a species of Chaetanthera
Ruiz & Pavon (Asteraceae). –
Taxon 39: 430-432.
Anderberg AA, Freire SE. 1991. A cladistic
and biogeographic analysis of the Lucilia group (Asteraceae, Gnaphalieae). – Bot. J.
Linn. Soc. 106: 173-198.
Anderberg AA, Källersjö M. 1988. The tribal
position of the genus Oedera L. (Compositae). – Bot. J. Linn. Soc.
96: 323-332.
Anderberg AA, Karis PO. 1995.
Psednotrichia, a genus of the tribe Senecioneae hitherto misplaced in
the Astereae (Asteraceae). –
Nord. J. Bot. 15: 375-379.
Anderberg AA, Pandey AK. 2008.
Nanothamnus sericeus Thomson, a derived species of Blumea.
– Compositae Newsl. 46: 8-19.
Anderberg AA, Karis PO, El-Ghazaly G. 1992.
Cratystylis, an isolated genus of the Asteraceae-Cichorioideae. – Aust.
Syst. Bot. 5: 81-94.
Anderberg AA, Eldenäs P, Bayer RJ, Englund
M. 2005. Evolutionary relationships in the Asteraceae tribe Inuleae (incl.
Plucheeae) evidenced by DNA sequences of ndhF; with notes on the
systematic positions of some aberrant genera. – Organisms Divers. Evol. 5:
135-146.
Anderberg AA, Baldwin BG, Bayer RG,
Breitwieser J, Jeffrey C, Dillon MO, Eldenäs P, Funk V, Garcia-Jacas N, Hind
DJN, Karis PO, Lack HW, Nesom G, Nordenstam B, Oberprieler C, Panero JL,
Puttock C, Robinson H, Stuessy TF, Susanna A, Urtubey E, Vogt R, Ward J, Watson
LE. 2006 [2007]. Compositae. – In: Kubitzki K, Kadereit JW, Jeffrey C (eds),
The families and genera of vascular plants VIII. Flowering plants. Eudicots.
Asterales, Springer, Berlin, Heidelberg, New York, pp. 61-588.
Anderberg AA, Ghahremaninejad F, Källersjö
M. 2007. The enigmatic genus Dipterocome. – Compositae Newsl. 45:
23-36.
Anderberg AA, Englund M, Beentje H. 2008. On
the systematic position of Inula rungwensis. – Compositae Newsl. 46:
83-84.
Anderson LC. 1964. Studies on
Petradoria (Compositae): anatomy, cytology, taxonomy. – Trans.
Kansas Acad. Sci. 66: 632-684.
Anderson LC. 1966. Cytotaxonomic studies in
Chrysothamnus (Astereae-Compositae). – Amer. J. Bot. 53: 204-212.
Anderson LC. 1970a. Studies on
Bigelowia (Astereae-Compositae) I. Morphology and taxonomy. – Sida
3: 451-465.
Anderson LC. 1970b. Floral anatomy of
Chrysothamnus (Astereae, Compositae). – Sida 3: 466-503.
Anderson LC. 1972. Studies on
Bigelowia (Astereae-Compositae) II. Xylary comparisons, woodiness, and
paedomorphosis. – J. Arnold Arbor. 53: 499-514.
Anderson LC. 1977. Studies on
Bigelowia (Astereae-Compositae) III. Cytotaxonomy and biogeography.
– Syst. Bot. 2: 209-218.
Anderson LC. 1987. Boltonia
apalachicolensis (Asteraceae): a new species from
Florida. – Syst. Bot. 12: 133-138.
Anderson LC. 1994. A revision of
Hasteola (Asteraceae) in
the New World. – Syst. Bot. 19: 211-219.
Anderson LC, Creech JB. 1975. Comparative
leaf anatomy of Solidago and related Asteraceae. – Amer. J. Bot. 62:
486-493.
Anderson LC, Weberg PS. 1974. The anatomy and
taxonomy of Vanclevea (Asteraceae). – Great Basin Natur.
34: 151-160.
Anderson LC, Kyhos DW, Mosquin T, Powell AM,
Raven PH. 1974. Chromosome numbers in the Compositae IX. Haplopappus
and other Astereae. – Amer. J. Bot. 61: 665-671.
Anderson LC, Hartman RL, Stuessy TF. 1979.
Morphology, anatomy, and taxonomic relationships of Otopappus
australis (Asteraceae). –
Syst. Bot. 4: 44-56.
Andrade Wagner M, Loeuille B, Siniscalchi C,
Melo-de-Pinna G, Parini J. 2014. Diversity of non-glandular trichomes in
subtribe Lychnophorinae (Asteraceae: Vernonieae) and taxonomic
implications. – Plant Syst. Evol. 300: 1219-1233.
Andrés-Sánchez S, Martínez-Ortega MM, Rico
E. 2014. Revisión taxonómica del género Bombycilaena (DC.) Smoljan.
(Asteraceae). – Candollea 69:
55-63.
Andrés-Sánchez S, Galbany-Casals M,
Bergmeier E, Rico E, Martínez-Ortega MM. 2015. Systematic significance and
evolutionary dynamics of the achene twin hairs in Filago (Asteraceae, Gnaphalieae) and related
genera: further evidence of morphological homplasy. – Plant Syst. Evol. 301:
1653-1668.
Andrus N, Trusty J, Santos-Guerra A, Jansen
RK, Francisco-Ortega J. 2004. Using molecular phylogenies to test
phytogeographical links between East/South Africa-southern Arabia and the
Macaronesian Islands: a review, and the case of Vierea and
Pulicaria section Vieraeopsis (Asteraceae). – Taxon 53:
333-346.
Angeles E, Folting K, Grieco PA, Huffman J,
Miranda R, Salmon M. 1982. Isolation and structure of stephalic acid, a new
clerodane diterpene from Stevia polycephala. – Phytochemistry 21:
1804-1806.
Angulo M, Dematteis M. 2014. Floral
microcharacters in Lessingianthus (Vernonieae, Asteraceae) and their taxonomic
implications. – Plant Syst. Evol. 300: 1925-1940.
Anke S, Niemüller D, Moll S, Hänsch R, Ober
D. 2004. Polyphyletic origin of pyrrolizidine alkaloids within the Asteraceae. Evidence from
differential tissue expression of homospermine synthase. – Plant Physiol.
136: 4037-4047.
Anthony J. 1926. A key to the genus
Codonopsis Wall. with a account of two undescribed species. – Notes
Roy. Bot. Gard. Edinburgh 15: 173-190.
Anthosen T. 1969. New chromenes from
Eupatorium species. – Acta Chem. Scand. 23: 3605-3607.
Antonelli A. 2008. Higher level phylogeny and
evolutionary trends in Campanulaceae subfam.
Lobelioideae: molecular signal overshadows morphology. – Mol. Phylogen. Evol.
46: 1-18.
Antonelli A. 2009. Have giant lobelias
evolved several times independently? Life form shifts and historical
biogeography of the cosmopolitan and highly diverse subfamily Lobelioideae (Campanulaceae). – BMC Biol. 7:
82.
Arano H. 1962. Cytological studies in
subfamily Carduoideae (Compositae) of Japan VIII. The karyotype analysis in
tribe Senecioneae. – Bot. Mag. (Tokyo) 75: 401-412.
Arano H. 1964. Cytological studies in
subfamily Carduoideae (Compositae) of Japan XVI. The karyotype analysis in
tribe Senecioneae. – Bot. Mag. (Tokyo) 77: 59-66.
Arano H. 1968. The karyotypes and the
geographical distribution in some groups of subfamily Carduoideae (Compositae)
of Japan. – Kromosomo 72/73: 2371-2388.
Arano H. 1970. The cytological studies in
subfamily Carduoideae of Compositae of Japan XXIII. The somatic chromosome
number in fifty species, four subspecies and six varieties. – J. Saitama
Univ., Nat. Sci. 18: 93-107.
Arano H. 1975. The cytogenetic studies in
subfamily Carduoideae of Japanese Compositae XXVIII. The karyotypes and the
speciations in the tribe Senecioneae. – J. Saitama Univ., Nat. Sci. 24:
15-29.
Arano H, Nakamura T. 1964. Cytological
studies in subfam. Carduoideae (Compositae) of Japan XV. The karyotype analysis
in some species of tribe Inuleae and Heliantheae. – Bot. Mag. (Tokyo) 77:
54-58.
Arano H, Saito H. 1979. The karyotypes and
chromosome evolution in family Campanulaceae (Japan) of
Asterales. – Kromosomo II: 433-447.
Ariza-Espinar L. 1969. Notas sobre Compositae
argentinas. – Kurtziana 5: 297-304.
Ariza-Espinar L. 1980. Las especies
centroargentinas de Hysterionica (Compositae). – Darwiniana 22:
537-549.
Ariza-Espinar L. 2000. Familia Asteraceae V. Tribu Heliantheae. –
Prodr. Flor. Fanerog. Argent. Centr. 2: 62-65.
Ariza-Espinar L, Cerana MM. 1986.
Contribucion al conocimiento de las especies de Stevia (Asteraceae) del centro de Argentina.
– Bol. Acad. Nac. Ci. Cordoba 57: 381-400.
Armbruster WS, Edwards ME, Debevec EM. 1994.
Floral character displacement generates assemblage structure of Western
Australian triggerplants (Stylidium). – Ecology 75: 315-329.
Armstrong JE. 2002. Fringe science: are the
corollas of Nymphoides (Menyanthaceae) flowers adapted for
surface tension interactions? – Amer. J. Bot. 89: 362-365.
Arnold ML, Jackson RC. 1978. Biochemical,
cytogenetic and morphological relationships of a new species of
Machaeranthera sect. Arida (Compositae). – Syst. Bot. 3:
208-217.
Arnold ML, Jackson RC. 1979. Genic
differentiation in Machaeranthera section Psilactis
(Compositae). – Southw. Natur. 24: 645-653.
Arriagada JE. 2003. Revision of the genus
Clibadium (Asteraceae,
Heliantheae). – Brittonia 55: 245-301.
Ascensão L, Pais MS. 1982. Secretory
trichomes of Artemisia crithmifolia. Some ultrastructural aspects. –
Bull. Soc. Bot. France 129, Act. Bot. Fr. 1: 83-87.
Ascensão L, Pais MS. 1985. Différenciation
et processus sécréteur des trichomes d’Artemisia campestris ssp.
maritima (Compositae). – Ann. Sci. Nat. Bot. 7: 149-179.
Ascensão L, Pais MS. 1987. Glandular
trichomes of Artemisia campestris (ssp. maritima): ontogeny
and histochemistry of secretory products. – Bot. Gaz. 148: 221-227.
Ascensão L, Pais MS. 1988. Ultrastructure
and histochemistry of secretory ducts in Artemisia campestris ssp.
maritima (Compositae). – Nord. J. Bot. 8: 283-292.
Aston HI. 1969. The genus Villarsia
(Menyanthaceae) in Australia.
– Muelleria 2: 3-63.
Aston HI. 1982. New Australian species of
Nymphoides Séguier (Menyanthaceae). – Muelleria 5:
35-51.
Aston HI. 1987. Nymphoides
beaglensis (Menyanthaceae): a new Australian
species. – Muelleria 6: 359-362.
Aston HI. 2003. Seed morphology of Australian
species of Nymphoides (Menyanthaceae). – Muelleria 18:
33-65.
Attar F, Ghahreman A. 2007. Cousinia
aligudarzensis (Asteraceae),
a new species of section Cynaroides Bunge from Iran. – Novon 17:
145-147.
Augier J, Rubat du Mérac M-L. 1951. La
phylogénie des Composées. – Rev. Sci. 89: 167-182.
Avetisian EM. 1964. K palinosistematike
nekotoryh rodov triby (Centaureinae) semejstva Asteraceae. – Trudy Bot. Inst.
Akad. Nauk Armjansk. S.S.R. 14: 31-47.
Avetisian EM. 1967. Pollen morphology of the
Campanulaceae and related
families. – Trudy Bot. Inst. Acad. Sci. Armenia 16: 5-41. [In Russian]
Avetisian EM. 1973. Palynology of the order
Campanulales s.l. – In:
Kuprianova LA (ed), Pollen and spore morphology of recent plants, Nauka,
Leningrad, pp. 90-93. [In Russian]
Avetisian EM. 1980. Pollen morphology of the
family Calyceraceae. – In:
Sistematika i Évolyutsiya Vysshikh Rastenii [Systematics and evolution of
higher plants], Nauka, Leningrad, pp. 57-64. [In Russian]
Avetisian EM. 1986. Pollen morphology of the
families Campanulaceae, Sphenocleaceae, and Pentaphragmataceae. – Bot.
Žurn. 71: 1003-1010. [In Russian with English summary]
Avetisian EM. 1988. Palynology of the
superorder Campanulanae. – Ph.D. diss., Institute of Botany, Yerevan,
Armenian SSR.
Ayers TJ. 1990. Systematics of
Heterotoma (Campanulaceae) and the evolution
of nectar spurs in the New World Lobelioideae. – Syst. Bot. 15: 296-327.
Ayers TJ. 1997. Three new species of
Lysipomia (Lobeliaceae) endemic to the páramos of southern Ecuador.
– Brittonia 49: 433-440.
Aytac Z, Anderberg AA. 2001. A new species of
Chrysophthalmum Schultz Bip. (Asteraceae-Inuleae) from Turkey. –
Bot. J. Linn. Soc. 137: 211-214.
Baagøe J. 1977a. Microcharacters in the
ligules of the Compositae. – In: Heywood VH, Harborne JB, Turner BL (eds),
The biology and chemistry of the Compositae, London, pp. 119-139.
Baagøe J. 1977b. Taxonomical application of
the ligule microcharacters in Compositae I. Anthemideae, Heliantheae, and
Tageteae. – Bot. Tidsskr. 71: 193-224.
Baagøe J. 1978. Taxonomical application of
the ligule microcharacters in Compositae II. Arctotideae, Astereae,
Calenduleae, Eremothamneae, Inuleae, Liabeae, Mutisieae, and Senecioneae. –
Bot. Tidsskr. 72: 125-147.
Baagøe J. 1980. SEM studies in ligules of
Lactuceae (Compositae). – Bot. Tidsskr. 75: 199-217.
Baas P. 1975. Vegetative anatomy and the
affinities of Aquifoliaceae,
Sphenostemon, Phelline, and Oncotheca. – Blumea
22: 311-407.
Babcock EB. 1947. The genus Crepis
I. The taxonomy, phylogeny, distribution and evolution of Crepis. –
Univ. Calif. Publ. Bot. 21: 1-197.
Babcock EB. 1947. The genus Crepis
II. Systematic treatment. – Univ. Calif. Publ. Bot. 22.
Babcock EB, Cameron DR. 1934. Chromosomes and
phylogeny in Crepis II. The relationships of one hundred eight
species. – Univ. Calif. Publ. Agric. Sci. 6: 287-324.
Babcock EB, Jenkins JA. 1943. Chromosomes and
phylogeny in Crepis III. The relationships of one hundred and thirteen
species. – Univ. Calif. Publ. Bot. 18: 241-291.
Babcock EB, Stebbins GL. 1938. The American
species of Crepis. Their interrelationships and distribution as
affected by polyploidy and apomixis. – Carnegie Inst. Washington Publ.
504.
Babcock EB, Stebbins GL. 1943. Systematic
studies of Cichoroideae. – Univ. Calif. Publ. Bot. 18: 227-240.
Babcock EB, Stebbins GL, Jenkins JA. 1937.
Chromosomes and phylogeny in some genera of the Crepidinae. – Cytologia,
Fujii Jubil. Vol.: 188-210.
Badillo VM. 1946. Contribución al
conocimiento de la sistmática y distribución geográfica de las compuestas en
Venezuela. – Bol. Venez. Ci. Nat. 10: 279-320.
Badillo VM. 1975. Un nuevo género de
Compositae para Venezuela. – Rev. Fac. Agron. (Maracay) 8: 187-191.
Badillo VM. 1992. Anotaciones adicionales al
género Tamananthus Badillo (Heliantheae-Asteraceae). – Ernstia 2: 17-19.
Badré F. 1o76. Campanulacées. – In: Flore
des Mascareignes 111, The Sugar Industry Research Institute, Mauritius.
Badré F, Cadet T. 1972. Lobeliaceae des
Mascareignes. – Adansonia, sér. II, 11: 667-683.
Badré F, Cadet T, Malplanche M. 1972. Étude
systématique et palynologique du genre Heterochaenia (Campanulaceae) endémique des
Mascareignes. – Adansonia, sér. II, 12: 267-278.
Baillon HE. 1891. Les Phelline de la
Nouvelle-Calédonie. – Bull. Mens. Soc. Linn. Paris 1: 937-939.
Bain JF. 1983. A biosystematic study of the
Senecio streptanthifolius Greene complex. – Ph.D. diss., University
of Alberta, Edmonton, Alberta.
Bain JF, Golden JL. 2000. A phylogeny of
Packera (Senecioneae; Asteraceae) based on Internal
Transcribed Spacer region sequence data and a broad sampling of outgroups. –
Mol. Phylogen. Evol. 2000: 331-338.
Bain JF, Jansen RK. 1995. A phylogenetic
analysis of the aureoid Senecio (Asteraceae) complex based on ITS
sequence data. – Plant Syst. Evol. 195: 209-219.
Bain JF, Jansen RK. 2006. A chloroplast DNA
hairpin structure provides useful phylogenetic data within tribe Senecioneae
(Asteraceae). – Can. J. Bot. 84:
862-868.
Bain JF, Tyson S, Bray DF. 1997. Variation in
pollen wall ultrastructure in New World Senecioneae (Asteraceae), with special reference
to Packera. – Can. J. Bot. 75: 730-735.
Bain JF, Walker J. 1995. A comparison of the
pollen wall ultrastructure of aureoid and non-aureoid Senecio species
(Asteraceae) in North America. –
Plant Syst. Evol. 195: 199-207.
Bain JF, Tyson BS, Bray DF. 1997. Variation
in pollen wall ultrastructure in New World Senecioneae (Asteraceae), with special reference
to Packera. – Can. J. Bot. 75: 730-735.
Baird KE, Funk VA, Wen J, Weeks A. 2010.
Molecular phylogenetic analysis of Leibnitzia Cass. (Asteraceae: Mutisieae:
Gerbera-complex), an Asian-North American disjunct genus. – J. Syst. Evol.
48: 161-174.
Baker EG. 1894. African species of
Lobelia § Rhynchopetalum. – J. Bot. 32: 65-70.
Bakshi Khaniki G. 1995. Meiotic studies on
some Iranian Centaurea (Compositae). – Cytologia 60: 341-346.
Baldwin BG. 1989. Chloroplast DNA
phylogenetic and biosystematic studies in Madiinae (Asteraceae). – Ph.D. diss.,
University of California, Davis, California.
Baldwin BG. 1992. Phylogenetic utility of the
internal transcribed spacers of nuclear ribosomal DNA in plants: an example
from the Compositae. – Mol. Phylogen. Evol. 1: 3-16.
Baldwin BG. 1993. Molecular phylogenetics of
Calycadenia (Compositae) based on ITS sequences of nuclear ribosomal
DNA: Chromosomal and morphological evolution re-examined. – Amer. J. Bot. 80:
222-238.
Baldwin BG. 1996. Phylogenetics of the
California tarweeds and the Hawaiian silversword alliance (Madiinae;
Heliantheae sensu lato). – In: Hind DJN, Beentje H (eds), Compositae:
systematics. Proceedings of the International Compositae Conference, Kew, 1994,
Royal Botanic Gardens, Kew, pp. 377-391.
Baldwin BG. 1997. Adaptive radiation of the
Hawaiian silversword alliance: congruence and incongruence of phylogenetic
evidence from molecular and non-molecular investigations. – In: Givnish T,
Sytsma K (eds), Molecular evolution and adaptive radiation, Cambridge
University Press, New York, pp. 103-128.
Baldwin BG. 1998. Evolution in the endemic
Hawaiian Compositae. – In: Stuessy TF, Ono M (eds), Evolution and speciation
of island plants, Cambridge University Press, Cambridge, pp. 49-73.
Baldwin BG. 1999a. New combinations in
Californian Arnica and Monolopia. – Novon 9: 460-461.
Baldwin BG. 1999b. New combinations and new
genera in the North American tarweeds (Compositae-Madiinae). – Novon 9:
462-471.
Baldwin BG. 2000. Constancea, a new
genus for Eriophyllum nevinii. – Madroño 46: 148-149.
Baldwin BG. 2003a. Characteristics and
diversity of Madiinae. – In: Carlquist S, Baldwin BG, Carr GD (eds), Tarweeds
& silverswords: evolution of the Madiinae (Asteraceae), Missouri Botanical
Garden Press, St. Louis, pp. 17-52.
Baldwin BG. 2003b. A phylogenetic perspective
on the origin and evolution of Madiinae. – In: Carlquist S, Baldwin BG, Carr
GD (eds), Tarweeds & silverswords: evolution of the Madiinae (Asteraceae), Missouri Botanical
Garden Press, St. Louis, pp. 193-228.
Baldwin BG. 2006. Contrasting patterns and
processes of evolutionary change in the tarweed-silversword lineage: revisiting
Clausen, Keck, and Hiesey’s findings. – Ann. Missouri Bot. Gard. 93:
64-93.
Baldwin BG. 2007. Adaptive radiation of
shrubby tarweeds (Deinandra) in the California Islands parallels
diversification of the Hawaiian silversword alliance (Compositae-Madiinae). –
Amer. J. Bot. 94: 237-248.
Baldwin BG. 2009. Heliantheae alliance. –
In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and
biogeography of Compositae, International Association for Plant Taxonomists,
Wien, pp. 689-711.
Baldwin BG. 2015. Nothoschkuhria, a
new South American genus for Schkuhria degenerica (Compositae,
Bahieae). – Phytoneuron 2015-56: 1-2.
Baldwin BG, Markos S. 1998. Phylogenetic
utility of the external transcribed spacer (ETS) of 18S-26S rDNA: congruence of
ETS and ITS trees of Calycadenia (Compositae). – Mol. Phylogen.
Evol. 10: 449-463.
Baldwin BG, Robichaux RH. 1995. Historical
biogeography and ecology of the Hawaiian silversword alliance: new molecular
phylogenetic perspectives. – In: Wagner WL, Funk VA (eds), Hawaiian
biogeography: evolution in a hot spot archipelago, Smithsonian Institution
Press, Washington, D.C., pp. 259-287.
Baldwin BG, Sanderson MJ. 1998. Age and rate
of diversification of the Hawaiian silversword alliance (Compositae). – Proc.
Natl. Acad. Sci. U.S.A. 95: 9402-9406.
Baldwin BG, Wessa BL. 2000a. Origin and
relationships of the tarweed-silversword lineage (Compositae-Madiinae). –
Amer. J. Bot. 87: 1890-1908.
Baldwin BG, Wessa BL. 2000b. Phylogenetic
placement of Pelucha and new subtribes in Helenieae sensu stricto
(Compositae). – Syst. Bot. 25: 522-538.
Baldwin BG, Wood KR. 2016. Origin of the Rapa
endemic genus Apostates: revisiting major disjunctions and
evolutionary conservatism in the Bahia alliance (Compositae: Bahieae). –
Taxon 1064-1080.
Baldwin BG, Kyhos DW, Dvořák J. 1990.
Chloroplast DNA evolution and adaptive radiation in the Hawaiian silversword
alliance (Asteraceae-Madiinae).
– Ann. Missouri Bot. Gard. 77: 96-109.
Baldwin BG, Kyhos DW, Dvořák J, Carr GD.
1991. Chloroplast DNA evidence for a North American origin of the Hawaiian
silversword alliance (Asteraceae).
– Proc. Natl. Acad. Sci. U.S.A. 88: 1840-1843.
Baldwin BG, Preston RE, Wessa BL, Wetherwax
M. 2001. A biosystematic and phylogenetic assessment of sympatric taxa in
Blepharizonia (Compositae-Madiinae). – Syst. Bot. 26: 184-194.
Baldwin BG, Wessa BL, Panero JL. 2002.
Nuclear rDNA evidence for major lineages of helenioid Heliantheae (Compositae).
– Syst. Bot. 27: 161-198.
Baldwin BG, Wessa BL, Panero JL. 2003.
Evolutionary insights from a putative taxonomic garbage can. Tribe Helenieae
revisited and revised. – Comp. Newslett. 40: 8.
Baltisberger M. 1993. Zytologische
Untersuchungen an Compositen aus Albanien. – Candollea 48: 437-448.
Baltisberger M, Lippert W. 1987. Compositen
aus Albanien. – Candollea 42: 679-691.
Bancheva S, Greilhuber J. 2006. Genome size
in Bulgarian Centaurea s.l. (Asteraceae). – Plant Syst. Evol.
257: 95-117.
Bancheva S, Raimondo MF. 2003. Biosystematic
studies of seven Balkan species from genus Cyanus (Compositae). –
Bocconea 16: 507-527.
Barclay HS, Earle FR. 1965. The search for
new industrial crops V. The South African Calenduleae (Compositae) as a source
of new oil seeds. – Econ. Bot. 19: 33-43.
Barghi N, Mugnier C, Šiljak-Yakovlev S.
1989. Karyological studies in some Hypochoeris spp. (Compositae) from
Sicily. – Plant Syst. Evol. 168: 49-57.
Barker MS, Kane NC, Matvienko M, Kozik A,
Michelmore RW, Knapp SJ, Rieseberg LH. 2008. Multiple paleopolyploidizations
during the evolution of the Compositae reveal parallel patterns of duplicate
gene retention after millions of years. – Mol. Biol. Evol. 25: 2445-2455.
Barker MS, Li Z, Kidder TI, Reardon CR, Lai
Z, Oliveira LO, Scascitelli M, Rieseberg LH. 2016. Most Compositae (Asteraceae) are descendants of a
paleohexaploid and all share a paleotetraploid ancestor with the Calyceraceae. – Amer. J. Bot.
103: 1203-1211.
Barkley TM. 1962. A revision of Senecio
aureus Linn. and allied species. – Trans. Kansas Acad. Sci. 65:
318-408.
Barkley TM. 1968a. Taxonomy of Senecio
multilobatus and its allies. – Brittonia 20: 267-284.
Barkley TM. 1968b. Intergradation of
Senecio sections Aurei, Tomentosi and
Lobati through Senecio mutabilis. – Southw. Natur. 13:
109-115.
Barkley TM. 1975. Senecio in
‘Flora of Panama’. – Ann. Missouri Bot. Gard. 62: 1244-1272.
Barkley TM. 1980. Taxonomic notes on
Senecio tomentosus and its allies. – Brittonia 32: 281-308.
Barkley TM. 1985. Generic boundaries in the
Senecioneae. – Taxon 34: 17-21.
Barkley TM. 1999. The segregates of
Senecio, s.l., and Cacalia, s.l., in the flora of North
America north of Mexico. – Sida 18: 661-672.
Barreda VD, Palazzesi L, Tellería MC. 2008.
Fossil pollen grains of Asteraceae
from the Miocene of Patagonia: Nassauviinae affinity. – Rev. Palaeobot.
Palynol. 151: 51-58..
Barreda VD, Palazzesi L, Tellería MC,
Katinas L, Crisci JV, Bremer K, Passala MG, Corsolini R, Rodríguez Brizuela R,
Bechis F. 2010. Eocene Patagonia fossils of the daisy family. – Science 329:
1621.
Barreda VD, Palazzesi L, Tellería MC,
Katinas L, Crisci JV. 2010. Fossil pollen indicates an explosive radiation of
basal asteracean lineages and allied families during Oligocene and Miocene
times in the Southern hemisphere. – Rev. Palaeobot. Palynol. 160: 102-110.
Barreda VD, Palazzesi L, Katinas L, Crisci
JV, Tellería MC, Bremer K, Passala MG, Bechis F, Corsolini R. 2012. An extinct
Eocene taxon of the daisy family (Asteraceae): evolutionary, ecological
and biogeographical implications. – Ann. Bot. 109: 127-134.
Barreda VD, Palazzesi L, Tellería MC,
Olivero EB, Raine JI. Forest F. 2015. Early evolution of the angiosperm clade
Asteraceae in the Cretaceous of
Antarctica. – Proc. Natl. Acad. Sci. U.S.A. 112: 10989-10994.
Barres L, Sanmartín I, Anderson CL, Susanna
A, Buerki S, Galbany-Casals M, Vilatersana R. 2013. Reconstructing the
evolution and biogeographic history of tribe Cardueae (Compositae). – Amer.
J. Bot. 100: 867-882.
Barreto JNV, Sancho G, Forte N. 2016. On the
presence of uncommon stylar glandular trichomes in Asteraceae: a study in
Kaunia R. M. King and H. Rob. (Oxylobinae, Eupatorieae). – Intern.
J. Plant Sci. 177: 760-770.
Barrett RL, Schmidt-Lebuhn AN, Thiele KR.
2013. Two Western Australian species of Ozothamnus transferred to
Pithocarpa (Asteraceae:
Gnaphalieae). – Nuytsia 23: 103-108.
Barrier M, Baldwin BG, Robichaux RH,
Purugannan MD. 1999. Interspecific hybrid ancestry of a plant adaptive
radiation: allopolyploidy of the Hawaiian silversword alliance (Asteraceae) inferred from floral
homeotic gene duplications. – Mol. Biol. Evol. 16: 1105-1113.
Barriera G. 2017. Novitates neocaledonicae.
VIII. Taxonomie et nomenclature du genre Phelline (Phellinaceae) avec la description
de la nouvelle espèce Phelline barrierei. – Candollea 72:
361-370.
Barriera G, Savolainen V, Spichiger R. 2006
[2007]. Phellinaceae. – In:
Kubitzki K, Kadereit JW, Jeffrey C (eds), The families and genera of vascular
plants VIII. Flowering plants. Eudicots. Asterales, Springer, Berlin,
Heidelberg, New York, pp. 608-610.
Barroso GM. 1969. Novitates compositarum II.
– Loefgrenia 36: 1-3.
Barroso GM. 1975. Baccharidiopsis
– um gênero novo da subtribo Baccharidinae Hoffmann (Tribo Astereae). –
Sellowia 27: 95-101.
Bartoli A, Tortosa RD. 2003. A new species of
Grindelia (Asteraceae,
Astereae) from the Mesetadel Somuncura (Patagonia), Argentina. – Brittonia
55: 146-149.
Bartoli A, Tortosa RD. 2012. Revision of the
North American species of Grindelia (Asteraceae). – Ann. Missouri Bot.
Gard. 98: 447-513.
Baruah NC, Sharma RP, Madhusudanan KP,
Thayagarajan G, herz W, Murari R. 1979. Sesquiterpene lactones of Tithonia
diversifolia. Stereo chemistry of the tagitinins and related compounds.
– J. Org. Chem. 44: 1831-1835.
Bässler M. 1963. Zur Taxonomie der Gattung
Achillea 1. Die Formenkreise um A. nobilis L. und A.
virescens (Fenzl) Heimerl. – Feddes Repert. 68: 139-162.
Batterman MRW, Lammers TG. 2004. Branched
foliar trichomes of Lobelioideae (Campanulaceae) and the
infrageneric classification of Centropogon. – Syst. Bot. 29:
448-458.
Bautista HP, Hind DJN. 2000. Two new species
of Acritopappus (Compositae: Eupatorieae: Ageratinae) from Bahia,
Brazil. – Kew Bull. 55: 949-956.
Bayer E. 1981. Revision der Gattung
Chrysocoma L. (Asteraceae-Astereae). – Mitt. Bot.
Staatssamml. München 17: 259-392.
Bayer RJ. 1984. Chromosome numbers and
taxonomic notes for North American species of Antennaria (Asteraceae: Inuleae). – Syst. Bot.
9: 74-83.
Bayer RJ. 1988. Patterns of isozyme variation
in western North American Antennaria (Asteraceae: Inuleae) I. Sexual
species of sect. Dioicae. – Syst. Bot. 13: 525-537.
Bayer RJ. 1989. Patterns of isozyme variation
in the Antennaria rosea (Asteraceae: Inuleae) polyploid agamic
complex. – Syst. Bot. 14: 389-397.
Bayer RJ. 1991. Allozymic and morphological
variation in Antennaria (Asteraceae: Inuleae) from the low
Arctic of northwestern North America. – Syst. Bot. 16: 492-506.
Bayer RJ. 2001. Xerochrysum Tzvelev,
a pre-existing generic name for Bracteantha Anderb. & Haegi (Asteraceae: Gnaphalieae). – Kew
Bull. 56: 1013-1015.
Bayer RJ, Cross EW. 2002. A reassessment of
tribal affinities of the enigmatic genera Printzia and Isoetopsis (Asteraceae), based on three
chloroplast sequences. – Aust. J. Bot. 50: 677-686.
Bayer RJ, Starr JR. 1998. Tribal phylogeny of
the Asteraceae based on two
non-coding chloroplast sequences, the trnL intron and
trnL/trnF intergenic spacer. – Ann. Missouri Bot. Gard. 85:
242-256.
Bayer RJ, Stebbins GL. 1982. A revised
classification of Antennaria (Asteraceae: Inuleae) of the Eastern
United States. – Syst. Bot. 7: 300-313.
Bayer RJ, Stebbins GL. 1987. Chromosome
numbers, patterns of distribution, and apomixes in Antennaria (Asteraceae: Inuleae). – Syst. Bot.
12: 305-319.
Bayer RJ, Soltis DE, Soltis PS. 1996.
Phylogenetic inferences in Antennaria (Asteraceae: Gnaphalieae: Cassiniinae)
based on sequences form nuclear ribosomal DNA internal transcribed spacers
(ITS). – Amer. J. Bot. 83: 516-527.
Bayer RJ, Puttock CF, Kelchner SA. 2000.
Phylogeny of South African Gnaphalieae (Asteraceae) based on two non-coding
chloroplast sequences. – Amer. J. Bot. 87: 259-272.
Bayer RJ, Greber DG, Bagnall NH. 2002.
Phylogeny of Australian Gnaphalieae (Asteraceae) based on chloroplast and
nuclear sequences, the trnL intron, trnL/trnF
intergenic spacer, matK, and ETS. – Syst. Bot. 27: 801-814.
Bayer RJ, Breitwieser I, Dillon M, Koekemoer
M, Ward J. 2003. Phylogeny of the Gnaphalieae based on three cpDNA sequences.
– Comp. Newslett. 40: 8.
Beaman JH. 1957. The systematics and
evolution of Townsendia (Compositae). – Contr. Gray Herb. 183:
1-151.
Beaman JH. 1991. Revision of
Hieracium (Asteraceae) in
Mexico and Central America. – Syst. Bot. Monogr. Missouri Bot. Gard. 29.
Beaman JH, Turner BL. 1962. Chromosome
numbers in Mexican and Guatemalan Compositae. – Rhodora 64: 271-276.
Bean AR. 1999a. Two new species of
Stylidium Willd. (Stylidiaceae) from north
Queensland. – Austrobaileya 5: 323-330.
Bean AR. 1999b. A revision of
Stylidium sect. Debilia Mildbr. S. sect.
Floodia Mildbr. and S. sect. Lanata A. R. Bean (Stylidiaceae). – Austrobaileya 5:
427-455.
Bean AR. 2000. A revision of
Stylidium subg. Andersonia (R. Br. ex G. Don) Mildbr. (Stylidiaceae). – Austrobaileya 5:
589-649.
Beauverd G. 1910a. Les espèces asiatiques de
genre Gerbera. – Bull. Soc. Bot. Genève, sér. II, 2: 28-49.
Beauverd G. 1910b. Contribution à l’étude
des Composées 3. Le grenre Cicerbita. – Bull. Soc. Bot. Genève,
sér. II, 2: 99-145.
Beauverd G. 1913a. Contribution à l’étude
des Composées C. – Noveaux Gerbera de la Section Anandria
O. Hoffm. – Bull. Soc. Bot. Genève, sér. II, 5: 147-149.
Beauverd G. 1913b. Contribution à l’étude
des Composées 8(1). Le genre Stuckertiella Beauverd. – Bull. Soc.
Bot. Genève 2: 205-209.
Beauverd G. 1913c. Contribution à l’étude
des Composées 8(2). Le genre Berroa extrait des Lucilia. –
Bull. Soc. Bot. Genève 2: 210-212.
Beauverd G. 1913d. Contribution à l’étude
des Composées 8(3). Le genre Facelis Cassini (emend. Beauverd). –
Bull. Soc. Bot. Genève 2: 212-220.
Beauverd G. 1913e. Contribution à l’étude
des Composées 8(4). Le genre Micropsis DC. (emend. Beauverd). –
Bull. Soc. Bot. Genève 2: 221-228.
Beauverd G. 1915. Contribution à l’étude
des Composées. – Bull. Soc. Bot. Genève, sér. II, 7: 21-56.
Beauverd G. 1922. Nouvelles Mutisiées des
Andes de Bolivia. – Bull. Soc. Bot. Genève, sér. II, 13: 10-11.
Beck JB, Nesom GL, Calie PJ, Baird GI, Small
RL, Schilling EE. 2004. Is subtribe Solidagininae (Asteraceae) monophyletic? – Taxon
53: 691-698.
Beentje HJ. 1999a. The genus
Tarchonanthus (Compositae-Mutisieae). – Kew Bull. 54: 81-95.
Beentje HJ. 1999b. A new genus and some new
species of Compositae from East Tropical Africa. – Kew Bull. 54: 97-102.
Beentje HJ. 2000a. Compositae (Part 1). –
In: Beentje HJ, Smith SAL (eds), Flora of tropical East Africa, A. A. Balkema,
Rotterdam, pp. 1-313.
Beentje HJ. 2000b. The genus
Brachylaena (Compositae: Mutisieae). – Kew Bull. 55: 1-41.
Beentje HJ. 2000c. New taxa and new
combinations in Helichrysum (Compositae: Inuleae). – Kew Bull. 55:
349-365.
Beentje HJ. 2002. Compositae (Part 2). –
In: Beentje HJ, Smith SAL (eds), Flora of tropical East Africa, A. A. Balkema,
Rotterdam, pp. 315-546.
Beentje HJ, Hind DJN. 2002. A new species of
Pseudoblepharispermum (Compositae, Inuleae: Plucheinae) from Somalia.
– Kew Bulletin 57: 213-217.
Behr O, Behr E, Zahn KH. 1939. Beiträge zur
Kenntnis der Hieracien von Südserben, Montenegro und Griechenland. – Glasn.
Skopsk. Naučn. Društva 20: 121-129.
Belcher RO. 1956. A revision of the genus
Erechtites with inquiries into Senecio and
Arrhenechtites. – Ann. Missouri Bot. Gard. 43: 1-85.
Belcher RO. 1989. Gynura
(Compositae) in Australia and Malesia, emended. – Kew Bull. 44: 533-542.
Belcher RO. 1992. The genus Senecio
(Compositae) on Lord Howe and Norfolk Islands. – Kew Bull. 47: 765-773.
Belcher RO. 1993. The ‘Senecio
aff. lautus’ complex (Asteraceae) in Australia I. Criteria
for exclusion of lautusoid Senecio of Australia from S.
lautus sensu stricto of New Zealand. – Aust. Syst. Bot. 6: 359-363.
Beliayev AA. 1984a. Seed anatomy in some
representatives of the family Campanulaceae. – Bot. Žurn. 69:
585-594. [In Russian]
Beliayev AA. 1984b. Surface ultrastructure
and some morphological characteristics of seeds in the representatives of the
family Campanulaceae. – Bot.
Žurn. 69: 890-898. [In Russian]
Beliayev AA. 1985. New data on the anatomical
structure of seed coat and ultrastructure of seed-surface of two
representatives of the genus Pentaphragma (Campanulaceae). – Bot. Žurn.
70: 955-957. [In Russian]
Bello MA, Álvarez I, Torices R,
Fuertes-Aguilar J. 2013. Floral development and evolution of capitulum
structure in Anacyclus (Anthemideae, Asteraceae). – Ann. Bot. 112:
1597-1612.
Beltran Santiago H, Galan de Mera A. 1996.
Senecio [sect. Senecio] ser. Lomincola nova y notas
corológicas y taxonómica sobre Senecio sect. Senecio (Asteraceae) para los Andes centrals
del Perú. – Bot. Complut. 21: 99-111.
Beltran Santiago H, Pruski JF. 2000.
Talamancalia y Rolandra (Asteraceae): dos nuevos registros
para el Peru. – Arnaldoa 7: 13-18.
Bengtson A, Anderberg AA. 2018. Species
diversification in the Mediterranean genus Chiliadenus (Inuleae-Asteraceae). – Plant Syst. Evol.
304: 853-860.
Bengtson A, Anderberg AA, Karis PO. 2011.
Phylogeny and generic delimitation of the Metalasia clade (Asteraceae-Gnaphalieae). – Intern.
J. Plant Sci. 172: 1067-1075.
Bengtson A, Anderberg AA, Karis PO. 2014.
Phylogeny and evolution of the South African genus Metalasia (Asteraceae-Gnaphalieae) inferred from
molecular and morphological data. – Bot. J. Linn. Soc. 174: 173-198.
Bengtson A, Englund M, Pruski JF, Anderberg
AA. 2017. Phylogeny of the Athroismeae (Asteraceae), with a new
circumscription of the tribe. – Taxon 66: 408-420.
Benko-Iseppon AM, Morawetz W. 2000.
Cytological comparison of Calyceraceae and Dipsacaceae with special
reference to their taxonomic relationships. – Cytologia 65: 123-128.
Bentham G. 1869. Notes on the stigmatic
apparatus of Goodeniaceae. –
Bot. J. Linn. Soc. 10: 203-206.
Bentham G. 1873. Notes on the classification,
history, and geographical distribution of Compositae. – Bot. J. Linn. Soc.
13: 335-577.
Bentham G. 1877. Notes on the gamopetalous
orders belonging to the campanulaceous and oleaceous groups. – J. Linn. Soc.
London 15: 1-16.
Bentley J, Klaassen ES, Bergh NG. 2015.
Philyrophyllum (Asteraceae) transferred from
Gnaphalieae to Athroismeae based on phylogenetic analysis of nuclear and
plastid DNA sequence data. – Taxon 64: 975-986.
Bentley J, Verboom GA, Bergh NG. 2017.
Species-level phylogenetic analysis on the Relhania clade of ”everlastings”
and a new generic treatment of species previously assigned to
Macowania and Arrowsmithia (Asteraceae: Gnaphalieae). – Taxon
66: 1421-1438.
Bergh NG, Linder HP. 2009. Cape
diversification and repeated out-of-southern-Africa dispersal in paper daisies
(Asteraceae-Gnaphalieae). – Mol.
Phylogen. Evol. 51: 5-18.
Bergh NG, McKenzie RJ. 2016. A revision of
Heterolepis Cass. (Asteraceae: Cichorioideae). – South
Afr. J. Bot. 105: 194-210.
Bergh NG, Trisos CH, Verboom GA. 2011.
Phylogeny of the ”Ifloga clade” (Asteraceae, Gnaphalieae), a lineage
occurring disjointly in the Northern and Southern Hemisphere, and inclusion of
Trichogyne in synonymy with Ifloga. – Taxon 60:
1065-1075.
Bergh NG, Haiden SA, Verboom GA. 2015.
Molecular phylogeny of the ’Cape snow’ genus Syncarpha (Asteraceae: Gnaphalieae) reveals a
need for generic re-delimitation. – South Afr. J. Bot. 100: 219-227.
Bergman B. 1935. Zytologische Studien über
die Fortpflanzung bei den Gattungen Leontodon und Picris. –
Svensk Bot. Tidskr. 29: 155-301.
Bergqvist G, Bremer B, Bremer K. 1992.
Chloroplast DNA restriction site variation and phylogenetic interrelationships
of some genera of the Heliantheae sensu lato (Asteraceae). – Nord. J. Bot. 12:
149-154.
Bergqvist G, Bremer B, Bremer K. 1995.
Chloroplast DNA variation and the tribal position of Eremothamnus (Asteraceae). – Taxon 44:
341-350.
Berry PE, Calvo RN. 1989. Wind pollination,
self-incompatibility and shifts in pollination systems in the high Andean genus
Espeletia (Asteraceae).
– Amer. J. Bot. 76: 1602-1614.
Besold B. 1971. Pollenmorphologische
Untersuchungen an Inuleen (Angianthinae, Relhaniinae, Athrixiinae). – Diss.
Bot. 14: 1-72.
Bhatnagar AK. 1973. Morphological and
embryological studies in Corokia. – Botanica (Delhi) 23: 149.
Bhattacharyya NK. 1972. Cytology of two
members of Campanulaceae and
Lobeliaceae and their interrelationships. – Cytologia 37: 435-443.
Bhuskute SM. 2002. Stylidium
tenellum Swartz (Stylidiaceae), a new record for
Maharashtra State. – J. Bombay Nat. Hist. Soc. 99: 374-375.
Bierner MW. 1978. The taxonomy of
Helenium sect. Cephalophora (Asteraceae). – Syst. Bot. 3:
277-298.
Bierner MW. 1989. Taxonomy of
Helenium sect. Amarum (Asteraceae). – Sida 13: 453-459.
Bierner MW. 1990. Present status of
Amblyolepis (Asteraceae:
Heliantheae). – Madroño 37: 133-140.
Bierner MW. 1994. Submersion of
Dugaldia and Plummera in Hymenoxys (Asteraceae: Heliantheae:
Gaillardiinae). – Sida 16: 1-8.
Bierner MW. 2001. Taxonomy of
Hymenoxys subgenus Picradenia and a conspectus of the
subgenera of Hymenoxys (Asteraceae: Helenieae:
Tetraneurinae). – Lundellia 4: 37-63.
Bierner MW, Jansen RK. 1998. Systematic
implications of DNA restriction site variation in Hymenoxys and
Tetraneuris (Asteraceae,
Helenieae, Gaillardiinae). – Lundellia 1: 17-26.
Bigazzi M. 1986. Ultrastructural and
cytochemical observations on fibrillar intranuclear inclusions in the family Campanulaceae. – Caryologia 39:
199-210.
Bingham RA, Ranker TA. 2000. Genetic
diversity in alpine and foothill populations of Campanula rotundifolia
(Campanulaceae). – Intern. J.
Plant Sci. 161: 403-411.
Binns SE, Baum BR, Arnason JT. 2002. A
taxonomic revision of Echinacea (Asteraceae: Heliantheae). – Syst.
Bot. 27: 610-632.
Bittmann M. 1990. Die Gattung
Adenocaulon (Compositae) 1: Morphologie. – Candollea 45: 389-420.
Blackmore S. 1981. Palynology and
intergeneric relationships in subtribe Hyoseridinae (Compositae: Lactuceae).
– Bot. J. Linn. Soc. 82: 1-13.
Blackmore S. 1982a. A functional
interpretation of Lactuceae (Compositae) pollen. – Plant Syst. Evol. 141:
153-168.
Blackmore S. 1982b. The apertures of
Lactuceae (Compositae) pollen. – Pollen Spores 24: 453-462.
Blackmore S. 1982c. Palynology of subtribe
Scorzonerinae (Compositae: Lactuceae) and its taxonomic significance. – Grana
21: 149-160.
Blackmore S. 1986. The identification and
taxonomic significance of lophate pollen in the Compositae. – Can. J. Bot.
64: 3101-3112.
Blackmore S, Barnes SH. 1988. Pollen ontogeny
in Catananche caerulea L. (Compositae: Lactuceae) I. Premeiotic phase
to establishment of tetrads. – Ann. Bot., N. S., 62: 605-614.
Blackmore S, Persson V. 1996. Palynology and
systematics of the Crepidinae (Compositae: Lactuceae). – In: Hind DJN,
Beentje HJ (eds), Compositae: systematics. Proceedings of the International
Compositae Conference, Kew, 1994, vol. 1, Royal Botanic Gardens, Kew, pp.
111-122.
Blackmore S, Helvoort HAM van, Punt W. 1984.
On the terminology, origins and functions of caveate pollen in Compositae. –
Rev. Palaeobot. Palyn. 43: 293-301.
Blackmore S, Wortley AH, Skvarla JJ, Robinson
H. 2009. Evolution of pollen in Compositae. – In: Funk VA, Susanna A, Stuessy
TF, Bayer RJ (eds), Systematics, evolution, and biogeography of Compositae,
International Association for Plant Taxonomists, Wien, pp. 101-130.
Blake SF. 1918. A revision of the genus
Viguiera. – Contr. Gray Herb. Harvard Univ. 54: 1-205.
Blake SF. 1921a. Revision of the genus
Acanthospermum. – Contr. U.S. Natl. Herb. 20: 383-392.
Blake SF. 1921b. Revision of the genus
Tithonia. – Contr. U.S. Natl. Herb. 20: 423-436.
Blake SF. 1922. Two new species of
Acanthospermum from the Galápagos Islands. – J. Washington Acad.
Sci. 12: 200-205.
Blake SF. 1924a. Hemibaccharis, a
new genus of Baccharidinae. – Contr. U.S. Natl. Herb. 20: 543-554.
Blake SF. 1924b. New American Asteraceae. – Contr. U.S. Natl.
Herb. 22: 587-661.
Blake SF. 1935. The genus
Chionopappus of Bentham (Asteraceae). – J. Washington Acad.
Sci. 25: 488-493.
Blake SF. 1937. Tracyina, a new
genus of Asteraceae from Northern
California. – Madroño 4: 73-77.
Blanca López G. 1980. Notas
cariosistemáticas en el género Centaurea L. sect.
Acrocentroides Willk. I. – An. Jard. Bot. Madrid 36: 349-369.
Blanca López G. 1981a. Notas
cariosistemáticas en el género Centaurea L. sect.
Willkommia G. Blanca II. Conclusiones. – An. Jard. Bot. Madrid 38:
109-125.
Blanca López G. 1981b. Revision del género
Centaurea L. sect. Willkommia G. Blanca, nom. nov. –
Lagascalia 10: 131-205.
Blionis GJ, Vokou D. 2002. Structural and
functional divergence of Campanula spatulata subspecies on Mt Olympos
(Greece). – Plant Syst. Evol. 232: 89-105.
Bloch C, Weiss-Schneeweiss H, Schneeweiss GM,
Barfuss MHJ, Rebernig CA, Villasenor JL, Stuessy TF. 2009. Molecular
phylogenetic analyses of nuclear and plastid DNA sequences support dysploid and
polyploid chromosome number changes and reticulate evolution in the
diversification of Melampodium (Millerieae, Asteraceae). – Mol. Phylogen. Evol.
53: 220-233.
Blöch C, Dickoré WB, Samuel R, Stuessy TF.
2010. Molecular phylogeny of the edelweiss (Leontopodium, Asteraceae-Gnaphalieae). –
Edinburgh J. Bot. 67: 235-264.
Bøcher TW. 1960. Experimental and
cytological studies on plants species V. The Campanula rotundifolia
complex. – K. Danske Vidensk. Selsk. Biol. Skr. 11: 1-69.
Bøcher TW. 1964. Chromosome connections and
aberrations in the Campanula persicifolia group. – Svensk Bot.
Tidskr. 58: 1-17.
Bohlmann F. 1990. Chemistry of the
Heliantheae (Compositae). – Mabry TJ, Wagenitz G (eds), Research advances in
the Compositae, Springer, Wien, pp. 67-75.
Bohlmann F, Fiedler L. 1978. Notiz über ein
neues Germacrolide aus Chromolaena glaberrima. – Chem. Ber. 111:
408.
Bohlmann F, Grenz M. 1975. Neue
Sesquiterpenlactone aus Athanasia-Arten. – Chem. Ber. 108:
357-361.
Bohlmann F, Grenz M. 1977. Über neue
Inhaltsstoffe aus Vertretern der Eupatorium-Gruppe. – Chem. Ber.
110: 1321-1329.
Bohlmann F, Grenz M. 1979. Neue
Tremeton-Derivate aus Doronicum macrophyllum. – Phytochemistry 18:
179-181.
Bohlmann F, Jakupovic J. 1990. Progress in
the chemistry of the Vernonieae (Compositae). – Plant Syst. Evol. [Suppl.] 4:
3-43.
Bohlmann F, Knoll KH. 1979. Neuartige
Sesquiterpenlactone und neue Acetylverbindungen aus Athanasia-Arten.
– Phytochemistry 18: 995-1001.
Bohlmann F, Rao N. 1972. Neue
Furansesquiterpene aus Athanasia-Arten. – Tetrahedron Lett. 1972:
1295-1296.
Bohlmann F, Suwita. 1979. Ein neues
Bisabolenderivat und ein neues Dihydrokaffeesäure-Derivat aus
Tarchonanthus trilobus. – Phytochemistry 18: 677-678.
Bohlmann F, Zdero C. 1972a. Zwei neue
Sesquiterpen-Lactone aus Lidbeckia pectinata Berg. und Pentzia
elegans DC. – Tetrahedron Letters 1972: 621-624.
Bohlmann F, Zdero C. 1972b. Ein neues
Furansesquiterpen aus Phymaspermum parvifolium. – Tetrahedron Lett.
1972: 851-852.
Bohlmann F, Zdero C. 1972c.
Leysseral-angelicat, ein neuartiges Benzofuran-Derivat. – Chem. Ber. 105:
2534-2538.
Bohlmann F, Zdero C. 1974a. Neue
Acetylverbindungen aus südafrikanischen Vertretern der Tribus Anthemideae. –
Chem. Ber. 107: 1044-1048.
Bohlmann F, Zdero C. 1974b. Über ein neues
Furansesquiterpen aus Stilpnophyton linifolium (Thunb.) Less. –
Chem. Ber. 107: 1071-1073.
Bohlmann F, Zdero C. 1977. Ein neues
Guajanolid aus Matricaria zuubergensis. – Phytochemistry 16:
136-137.
Bohlmann F, Zdero C. 1978a. New furane
sesquiterpenes from Eumorphia species. – Phytochemistry 17:
1155-1159.
Bohlmann F, Zdero C. 1978b. New
sesquiterpenes and acetylenes from Athanasia and Pentzia
species. – Phytochemistry 17: 1595-1599.
Bohlmann F, Zdero C. 1982. Sesquiterpene
lactones from Inezia integrifolia. – Phytochemistry 21:
2743-2745.
Bohlmann F, Arndt C, Bornowski H, Kleine K-M,
Herbst P. 1964. Neue Acetylenverbindungen aus Chrysanthemum-Arten. –
Chem. Ber. 97: 1179-1192.
Bohlmann F, Zdero C, Grenz M. 1977a. Weitere
Inhaltsstoffe aus südafrikanischen Senecio-Arten. – Chem. Ber. 110:
474-486.
Bohlmann F, Zdero C, Grenz M. 1977b. Weitere
Inhaltsstoffe aus Vertretern der Eupatorium-Gruppe. – Chem. Ber.
110: 1034-1041.
Bohlmann F, Jakupovic J, Lonitz M. 1977.
Über Inhaltsstoffe der Eupatorium-Gruppe. – Chem. Ber. 110:
301-314.
Bohlmann F, Mahanta PK, Suwita A, Suwita An,
Anant A, Zdero C, Dorner W, Eilers D, Grenz M. 1977. Neue Sesquiterpenelactone
und andere Inhaltsstoffe aus Vertretern der Eupatorium-Gruppe. –
Phytochemistry 16: 1973-1981.
Bohlmann F, Suwita A, Mabry TJ. 1978. New
labdane derivatives and further constituents of Brickellia species.
– Phytochemistry 17: 763-765.
Bohlmann F, Dutta LN, Dorner W, King RM,
Robinson H. 1979. Zwei neue Guajanolide sowie weitere Longipinenester aus
Stevia-Arten. – Phytochemistry 18: 673-675.
Bohlmann F, Zdero C, King RM, Robinson H.
1979. New prenylflavanoids from Marshallia grandiflora. –
Phytochemistry 18: 1246-1247.
Bohlmann F, Dutta LN, King RM, Robinson H.
1979. Neue Sesquiterpenlactone aus Eupatorium sessilifolium. –
Phytochemistry 18: 1401-1403.
Bohlmann F, Suwita A, King RM, Robinson H.
1980. Neue ent-labdan-derivate aus Austroeupatorium
chaparense. – Phytochemistry 19: 111-114.
Bohlmann F, Dutta LN, Kerr K. 1980.
Sesquiterpenlactone aus Oxylobus oaxacanus. – Phytochemistry 19:
691-692.
Bohlmann F, Zdero C, King RM, Robinson H.
1980. New helangiolides from Conocliniopsis prasiifolia. –
Phytochemistry 19: 1547-1549.
Bohlmann F, Zdero C, Robinson H, King RM.
1980. Guaianolides from Agrianthus pungens. – Phytochemistry 19:
1873-1874.
Bohlmann F, Jakupovic J, Dhar AK, King RM,
Robinson H. 1981. Heliangolides and diterpenes from Hartwrightia
floridana. – Phytochemistry 20: 843-345.
Bohlmann F, Zdero C, King RM, Robinson H.
1981. Germacrolides, a guaianolide with a β-lactone ring and further
constituents from Grazielia species. – Phytochemistry 20:
1069-1075.
Bohlmann F, Dhar AK, Jakupovic J, King RM,
Robinson H. 1981. Two sesquiterpene lactones with an additional propiolactone
ring from Disynaphia halimifolia. – Phytochemistry 20: 1077-1080.
Bohlmann F, Zdero C,Fiedler L, Robinson H,
King RM. 1981. A labdane derivative from Chromolaena collina and a
P-hydroxyacetophenone derivative from Stomatanthes corumbensis. –
Phytochemistry 20: 1141-1143.
Bohlmann F, Zdero F, Pickard J, Robinson H,
King RM. 1981. New types of sesquiterpene lactones and other constituents from
Trichogonia species. – Phytochemistry 20: 1323-1333.
Bohlmann F, Gupta RK, King RM, Robinson H.
1981. Prostaglandin-like fatty acid derivative from Chromolaena morii.
– Phytochemistry 20: 1417-1418.
Bohlmann F, Jakupovic J, King RM, Robinson H.
1981. New germacranolides, guaianolides and rearranged guaianolides from
Lasiolaena santosii. – Phytochemistry 20: 1613-1622.
Bohlmann F, Abraham W-R, Robinson H, King RM.
1981. Heliangiolides and other constituents from Bejaranoa
semistriata. – Phytochemistry 20: 1639-1642.
Bohlmann F, Suwita A, Robinson H, King RM.
1981. Six guaianolides from Stylotrichium rotundifolium. –
Phytochemistry 20: 1887-1890.
Bohlmann F, Ahmed M, King RM, Robinson H.
1981. A germacrolide and a longipinene derivative from Eupatoriadelphus
purpureus. – Phytochemistry 20: 2027-2028.
Bohlmann F, Kramp W, Gupta RK, King RM,
Robinson H. 1981. Four guaianolides and other constituents from three
Kaunia species. – Phytochemistry 20: 2375-2378.
Bohlmann F, Zdero C, King RM, Robinson H.
1982. Humulene derivatives from Acritopappus prunifolius. –
Phytochemistry 21: 147-150.
Bohlmann F, Jakupovic J, Schuster A, King RM,
Robinson H. 1982. Guaianolides and homoditerpenes from Lasiolaena
morii. – Phytochemistry 21: 161-165.
Bohlmann F, Adler A, King RM, Robinson H.
1982. Ent-labdanes from Mikania alvimii. – Phytochemistry
21: 173-176.
Bohlmann F, Bapugi M, Jakupovic J, King RM,
Robinson H. 1982. Unusual diterpenes from Brickellia eupatoiroides.
– Phytochemistry 21: 181-186.
Bohlmann F, Singh P, jakupovic J, King RM,
Robinson H. 1982. Three cadinene derivatives and a prostaglandin-like acid from
Chromolaena species. – Phytochemistry 21: 371-374.
Bohlmann F, Ahmed M, Jakupovic J, King RM,
Robinson H. 1982. Labdane and dihydronerolidol derivatives from Brickellia
diffusa. – Phytochemistry 21: 691-694.
Bohlmann F, Singh P, King RM, Robinson H.
1982. New guaianolides from Pseudostifftia kingii. – Phytochemistry
21: 1171-1172.
Bohlmann F, Adler A, Jakupovic J, King RM,
Robinson H. 1982. A dimeric germacrolide and other sesquiterpene lactones from
Mikania species. – Phytochemistry 21: 1349-1355.
Bohlmann F, Zdero C, King RM, Robinson H.
1983. 8-β-Tigloylguaiagrazielolide from Campovassouria bupleurifolia.
– Phytochemistry 22: 2860-2862.
Bohlmann F, Zdero C, King RM, Robinson H.
1984. Heliangiolides and bejaranolides from Conocliniopsis
prasiifolia. – Phytochemistry 23: 1509-1511.
Bohlmann F, Zdero C, Jakupovic J, Gerke T,
Wallmeyer M, King RM, Robinson H. 1984. Neue Sesquiterpenlactone und
Rosan-Derivate aus Trichogonia-Arten. – Liebigs Ann. Chem. 1984:
162-185.
Bohm BA. 1977. Helenieae (sensu Bentham) –
chemical review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology
and chemistry of the Compositae II, Academic Press, London, pp. 739-767.
Bohm BA, Stuessy TF. 1995. Flavonoid
chemistry of Barnadesioideae (Asteraceae). – Syst. Bot. 20:
22-27.
Bohm BA, Stuessy TF. 2001. Flavonoids of the
sunflower family (Asteraceae). –
Springer, Berlin, Heidelberg, New York.
Bohm BA, Nicholls KW, Ornduff R. 1986.
Flavonoids of the Menyanthaceae: intra- and
interfamilial relationships. – Amer. J. Bot. 73: 204-213.
Bohm BA, Reid A, DeVore M, Stuessy TF. 1995.
Flavonoid chemistry of Calyceraceae. – Can. J. Bot. 73:
1962-1965.
Bolick MR. 1977. Observations on style branch
exposure in Eupatorium havanense H. B. K. (Eupatorieae-Compositae).
– Taxon 26: 239-240.
Bolick MR. 1978a. A light and electron
microscopic study of the pollen of the Vernonieae (Compositae). – Ph.D.
diss., University of Texas, Austin, Texas.
Bolick MR. 1978b. Taxonomic, evolutionary,
and functional considerations of Compositae pollen ultrastructure and
sculpture. – Plant Syst. Evol. 130: 209-218.
Bolick MR. 1983a. A cladistic analysis of the
Ambrosiinae Less. and Engelmanniinae Stuessy. – In: Funk VA, Platnick N
(eds), Advances in cladistics, Columbia University Press, New York, pp.
125-141.
Bolick MR. 1983b. Exine structure of
Trichospira verticillata (L.) Blake (Compositae) and its implications
for the tribal placement of the genus. – Amer. J. Bot. 70: 463-465.
Bolick MR. 1985. Cladistic analysis of
Iva: a case in point. – Taxon 34: 81-84.
Bolick MR. 1991a. Pollen diameter, exine
thickness, and ultrastructure type in the tribes of the Compositae. –
Compositae Newsl. 19: 17-21.
Bolick MR. 1991b. Systematics of
Salmea DC. (Compositae: Heliantheae). – Syst. Bot. 16: 462-477.
Bolick MR, Skvarla JJ. 1976. A reappraisal of
the pollen ultrastructure of Parthenice mollis Gray (Compositae). –
Taxon 25: 261-264.
Bolick MR, Skvarla JJ, Turner BL, Patel VA,
Tomb AS. 1984. On cavities in spines of compositae pollen – a taxonomic
perspective. – Taxon 33: 289-293.
Bond P. 1987. A revision of
Oldenburgia (Asteraceae-Mutisieae). – South Afr.
J. Bot. 53: 493-500.
Bonifacino JM. 2005. Nardophyllum
cabrerae (Asteraceae:
Astereae), a new species from Argentina and new taxonomic changes involving
Nardophyllum Hook. et Arn. – Taxon 54: 688-692.
Bonifacino JM. 2008. Reinstatement of
Ocyroe (Compositae: Astereae). – Brittonia 60: 205-212.
Bonifacino JM. 2009. Taxonomic revision of
the Chiliotrichum group sensu stricto (Compositae: Astereae). –
Smithsonian Contributions to Botany 92: 1-128.
Bonifacino JM, Funk VA. 2012. Phylogenetics
of the Chiliotrichum group (Compositae: Astereae): the story of the
fascinating radiation in the paleate Astereae genera from southern South
America. – Taxon 61: 180-196.
Bonifacino JM, Sancho G. 2004.
Guynesomia (Asteraceae:
Astereae), a new genus from Central Chile. – Taxon 53: 673-678.
Bonifacino JM, Sancho G, Marchesi EH. 2009. A
new combination in Asteropsis (Compositae: Astereae), and a synopsis
of the genus. – Brittonia 61: 1-7.
Borgen L. 1972. Embryology and achene
morphology in endemic Canarian species of Chrysanthemum (L.) Hoffm.
subgenus Argyranthemum (Webb) Harling. – Norw. J. Bot. 19:
149-170.
Borgen L. 1980. A new species of
Argyranthemum (Compositae) from the Canary Islands. – Norw. J. Bot.
27:163-165.
Borgen L, Leitch I, Santos-Guerra A. 2003.
Genome organization in diploid hybrid species of Argyranthemum (Asteraceae) in the Canary Islands.
– Bot. J. Linn. Soc. 141: 491-501.
Borhidi A, Gondár E, Kiss T, Orosz-Kovács
Z. 1992 [1993 or 1994]. Ekmaniopappus Borhidi gen. novum (Senecioneae,
Asteraceae) in Hispaniola. –
Acta Bot. Hung. 37: 105-117.
Borsch T, Korotkova N, Raus T, Lobin W,
Löhne C. 2009. The petD group II intron as a species level marker:
utility for tree inference and species identification in the diverse genus
Campanula (Campanulaceae). – Willdenowia
39: 7-33.
Bosque MEQ del, López-Flores I,
Suárez-Santiago V, Garrido-Ramos MA. 2014. Satellite-DNA diversification and
the evolution of major lineages in Cardueae (Carduoideae Asteraceae). – J. Plant Res. 127:
575-583.
Boulos L. 1959. Cytotaxonomic studies in the
genus Sonchus 1. Sonchus gigas Boulos nov. spec., a new
tetraploid Egyptian species. – Bot. Not. 112: 363-368.
Boulos L. 1960a. Cytotaxonomic studies in the
genus Sonchus, preparation for a monograph. – M.Sc. thesis, Faculty
of Science, Cairo University, Egypt.
Boulos L. 1960b. Cytotaxonomic studies in the
genus Sonchus 2. The genus Sonchus, a general systematic
treatment. – Bot. Not. 113: 400-420.
Boulos L. 1961. Cytotaxonomic studies in the
genus Sonchus 3. On the cytotaxonomy and distribution of Sonchus
arvensis L. – Bot. Not. 114: 57-64.
Boulos L. 1962a. Cytotaxonomic studies in the
genus Sonchus 4. The generic status of some species earlier treated as
Sonchus. – Bot. Not. 115: 58-60.
Boulos L. 1962b. Sur la taxonomie de
Sonchus Bipontini Aschers. – Bull. Jard. Bot. État, Bruxelles 32:
105-106.
Boulos L. 1965. Babcockia, un
nouveau genre de Composées des Îles Canaries. – Bull. Jard. Bot. État,
Bruxelles 35: 63-66.
Boulos L. 1967a. Nomenclatural changes and
new taxa in Sonchus from the Canary Islands. – Nytt Mag. Bot. 14:
7-18.
Boulos L. 1967b. Taeckholmia, a new
genus of Compositae from Canary Islands. – Bot. Not. 120: 95-108.
Boulos L. 1967c. Sonchus friesii, a
new African species of Compositae. – Bot. Not. 120: 456-459.
Boulos L. 1968. The genus Sonchus
and allied genera in the Canary Islands. – Cuadernos Bot. 3: 19-26.
Boulos L. 1972. Révision systématique du
genre Sonchus L. s.l. I. Introduction et classification. – Bot. Not.
125: 287-305.
Boulos L. 1973. Révision systématique du
genre Sonchus L. s.l. IV. Sous-genre 1. Sonchus. – Bot.
Not. 126: 155-196.
Boulos L. 1974a. Révision systématique du
genre Sonchus L. s.l. V. Sous-genre 2. Dendrosonchus. –
Bot. Not. 127: 7-37.
Boulos L. 1974b. Révision systématique du
genre Sonchus L. s.l. VI. Sous-genre 3. Origosonchus. Genres
Embergeria, Babcockia et Taeckholmia. Species
exclusae et dubiae. Index. – Bot. Not. 127: 402-451.
Boulos L, Jeffrey C. 1969. Sonchus
gigas and S. macrocarpus (Compositae). – Taxon 18: 348-349.
Boulter D, Gleaves JT, Haslett BG, Peacock D,
Jensen U. 1978. The relationships of 8 tribes of the Compositae as suggested by
plastocyanin amino acid sequence data. – Phytochemistry 17: 1585-1589.
Bramwell D, Dakshini KMM. 1971.
Luteolin-7-glucoside and hydroxycoumarins in Canary Islands Sonchus
species. – Phytochemistry 10: 2245-2246.
Bräutigam S, Greuter W. 2007. A new
treatment of Pilosella for the Euro-Mediterranean flora. –
Willdenowia 37: 123-137.
Breitwieser I, Podlech D. 1986. Die Gattung
Centaurea L. Sect. Willkommia G. Blanca in Nord-Afrika. –
Mitt. Bot. Staatssamml. München 22: 21-96.
Breitwieser I, Ward JM. 2003. Phylogenetic
relationships and character evolution in New Zealand and selected Australian
Gnaphalieae (Compositae) inferred from morphological and anatomical data. –
Bot. J. Linn. Soc. 141: 183-203.
Breitwieser I, Glenny D, Thorne A, Wagstaff
SJ. 1999. Phylogenetic relationships in Australasian Gnaphalieae (Compositae)
inferred from ITS sequences. – New Zealand J. Bot. 37: 399-412.
Bremer K. 1972. The genus Osmitopsis
(Compositae). – Bot. Not. 125: 9-48.
Bremer K. 1976a. New taxa of Osmitopsis
(Compositae). – Bot. Not. 129: 21-24.
Bremer K. 1976b. The genus Rosenia
(Compositae). – Bot. Not. 129: 97-111.
Bremer K. 1976c. The genus Relhania
(Compositae). – Opera Bot. 40: 1-86.
Bremer K. 1977. The genus Peyrousea
(Compositae). – Bot. Not. 130: 493-497.
Bremer K. 1978a. The genus Leysera
(Compositae). – Bot. Not. 131: 369-383.
Bremer K. 1978b. Oreoleysera and
Antithrixia, new and old South African genera of the Compositae. –
Bot. Not. 131: 449-453.
Bremer K. 1983. Taxonomy of Asaemia
with notes on Stilpnophyton (Compositae-Anthemideae). – Nord. J.
Bot. 3: 193-195.
Bremer K. 1987. Tribal interrelationships of
the Asteraceae. – Cladistics 3:
210-253.
Bremer K. 1988. A new corolla type from the
Asteraceae-Arctotideae. –
Compositae Newsl. 15: 12-16.
Bremer K. 1993a. New subtribes of the
Lactuceae (Asteraceae). – Novon
3: 328-330.
Bremer K. 1993b. Intercontinental
relationships of African and South American Asteraceae – a cladistic
biogeographic analysis. – In: Goldblatt P (ed), Biological relationships
between Africa and South America, Yale University Press, New Haven,
Connecticut, pp. 104-135.
Bremer, K (ed). 1994. Asteraceae: cladistics and
classification. – Timber Press, Portland, Oregon.
Bremer K. 1996. Major clades and grades of
the Asteraceae. – In: Hind DJN,
Beentje HJ (eds), Compositae: systematics. Proceedings of the International
Compositae Conference, Kew, 1994, vol. 1, Royal Botanic Gardens, Kew, pp.
1-7.
Bremer K, Gustafsson MHG. 1997. East Gondwana
ancestry of the sunflower alliance of families. – Proc. Natl. Acad. Sci.
U.S.A. 94: 9188-9190.
Bremer K, Humphries CJ. 1993. Generic
monograph of the Asteraceae-Anthemideae. – Bull.
Brit. Mus. (Nat. Hist.) Bot. 23: 71-177.
Bremer K, Jansen RK. 1992. A new subfamily of
the Asteraceae. – Ann. Missouri
Bot. Gard. 79: 414-415.
Bremer K, Källersjö M. 1985. Taxonomic
notes on Hymenolepis (Asteraceae-Anthemideae). – Nord. J.
Bot. 5: 517-520.
Bremer K, Jansen RK, Karis PO, Källersjö M,
Keeley SC, Kim K-J, Michaels HJ, Palmer JD, Wallace RS. 1992. A review of the
phylogeny and classification of the Asteraceae. – Nord. J. Bot. 12:
141-148.
Bremer K, Anderberg AA, karis PO, Lundberg J.
1994. Tribe Eupatorieae. – In: Bremer K (ed), Asteraceae. Cladistics and
classification, Timber Press, Portland, Oregon, pp. 625-680.
Bremer K, Eklund H, Medhanie G, Heidmersson
S, Laurent N, Maad J, Niklasson J, Nordin A. 1996. On the delimitation of
Matricaria versus Microcephala (Asteraceae: Anthemideae). – Plant
Syst. Evol. 200: 263-271.
Briquet J. 1916a. Étude carpologiques sur
les genre de Composées Anthemis, Ormenis et
Santolina. – Ann. Cons. Jard. Bot. Genève 18-19: 157-313.
Briquet J. 1916b. Carpologie comparée des
Santolines et des Achillées. – Arch. Sci. Phys. Nat., sér. IV, 41:
239-242.
Briquet J. 1916c. Organisation florale et
carpologie de l’Achillea fragrantissima (Forssk.) Boiss. – Arch.
Sci. Phys. Nat., sér. IV, 41: 242-245.
Brock TCM, Bongaerts MCM, Heijnen GJMA,
Heijthuijsen JHFG. 1983. Nitrogen and phosphorus accumulation and cycling by
Nymphoides peltata (Gmel.) O. Kuntze (Menyanthaceae). – Aquatic Bot.
17: 189-214.
Bronckers F, Stainer F. 1972. Contribution à
l’étude morphologique du pollen de la famille des Stylidiaceae. – Grana 12:
1-22.
Brook JP. 1951. Vegetative anatomy of
Carpodetus serratus Forst. – Trans. Proc. Roy. Soc. New Zealand 79:
276-285.
Brough P. 1927. Studies in the Goodeniaceae I. The life history of
Dampiera stricta R. Br. – Proc. Linn. Soc. New South Wales 52:
471-498.
Brouillet L, Lowrey TK, Urbatsch L,
Karaman-Castro V, Sancho G, Wagstaff S, Semple JC. 2009. Astereae. – In: Funk
VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and
biogeography of Compositae, International Association for Plant Taxonomists,
Wien, pp. 589-629.
Brouillet L, Anderberg AA, Nesom GL, Lowrey
TK, Urbatsch LE. 2009. Welwitschiella is a member of the African
subtribe Grangeinae (Asteraceae
– Astereae): a new phylogenetic position based on ndhF and ITS
sequence data. – Kew Bull. 64: 645-660.
Brown GK, Clark WD. 1981. Chromosome numbers
in South American Haplopappus Cass. (Compositae). – Amer. J. Bot.
68: 1218-1221.
Brown GK, Clark WD. 1982. Taxonomy of
Haplopappus sect. Gymnocoma (Compositae). – Syst. Bot. 7:
199-213.
Brown NE. 1913. The wahlenbergias of
Australia and New Zealand. – Gard. Chron., Ser. III, 54: 316-317, 336-337.
Bruce EA. 1934. The giant Lobelias of East
Africa. – Kew Bull. 1934: 61-88, 274.
Bruhl JJ, Quinn CJ. 1990. Cypsela anatomy in
the ‘Cotuleae’ (Asteraceae-Anthemideae). – Bot. J.
Linn. Soc. 102: 37-59.
Bruhl JJ, Quinn CJ. 1991. Floral morphology
and a reassessment of affinities in the ‘Cotuleae’ (Asteraceae). – Aust. Syst. Bot. 4:
637-654.
Brullo S. 1979. Taxonomic and nomenclatural
notes on the genera Jasonia Cass. and Chiliadenus Cass.
(Compositae). – Webbia 34: 289-308.
Brullo S, Marco G de. 2000. Taxonomical
revision of the genus Dittrichia (Asteraceae). – Portugaliae Acta
Biol. 19: 341-354.
Brullo S, Marcenò C, Pavone P. 1981.
Jasione sphaerocephala sp. nov. (Campanulaceae) from Italy. –
Nord. J. Bot. 1: 137-139.
Brunerye L. 1969. Les Senecio du
groupe Helenitis. – Ph.D. diss., Université de Rouen, France.
Bunwong S, Chantaranothai P. 2008. Pollen
morphology of the tribe Vernonieae (Compositae) in Thailand. – Nat. Hist. J.
Chulalongkorn Univ. 8: 45-55.
Bunwong S, Chantaranothai P, Keeley SC. 2014.
Revision and key to the Vernonieae (Compositae) of Thailand. – PhytoKeys 37:
25-101.
Burbidge AH. 1984. Breeding systems in
triggerplants. – Ph.D. diss., University of Western Australia, Nedlands,
Australia.
Burbidge AH, James SH. 1991. Postzygotic seed
abortion in the genetic system of Stylidium (Angiospermae: Stylidiaceae). – J. Heredity 82:
219-228.
Burbidge NT. 1982. A revision of
Vittadinia A. Rich. (Compositae) together with reinstatement of
Eurybiopsis DC. and description of a new genus, Camptacra.
– Brunonia 5: 1-72.
Burkart A. 1944. Estudio del género de
compuestas Chaptalia con especial referencia a las espécies
Argentinas. – Darwiniana 6: 505-594.
Burns GP. 1900. Beiträge zur Kenntnis der Stylidiaceae. – Flora 87:
313-354.
Burtt BL. 1949. Studies in the Ericales IX. The taxonomic
position of Wittsteinia. – Kew Bull. 3: 493-495.
Burtt BL. 1961. Compositae and the study of
functional evolution. – Trans. Bot. Soc. Edinb. 39: 216-232.
Burtt BL, Grau J. 1972. An extension of the
genus Macowania (Compositae). – Notes Roy. Bot. Gard. Edinb. 31:
373-376.
Burtt BL, Sunding P. 1973. Helichrysum
monogynum, a new species from Lanzarote, Canary Islands. – Bot. Not.
126: 340-344.
Buss CC, Lammers TG, Wise RR. 2001. Seed coat
morphology and its systematic implications in Cyanea and other genera
of Lobelioideae (Campanulaceae). – Amer. J. Bot.
88: 1301-1308.
Buttrose MS, Lott JNA. 1978. Inclusions in
seed protein bodies in members of the Compositae and Anacardiaceae:
comparison with other dicotyledonous families. – Can. J. Bot. 56:
2062-2071.
Cabrera Al. 1935. Mutisieas Argentinas nuevas
o interesantes. – Notas Mus. La Plata, Bot. 1: 55-69.
Cabrera AL. 1936. Las especies Argentinas y
Uruguayas del género Trixis. – Rev. Mus. La Plata, Secc. Bot., n.
s., 1: 31-86.
Cabrera AL. 1937. Revisión del género
Chaetanthera (Compositae). – Rev. Mus. La Plata, Secc. Bot., n. s.,
1: 87-210.
Cabrera AL. 1939. Las especies Tucumanas del
genero Senecio (Compositae). –Lilloa 5: 65-120.
Cabrera AL. 1946. El género
Hysterionica en el Uruguay en y la república Argentina. – Notas
Mus. La Plata, Bot. 11: 349-359.
Cabrera AL. 1949. El género Senecio
en Chile. – Lilloa 15: 27-501.
Cabrera AL. 1950. Observaciones sobre los
géneros Gocnatia y Moquinia. – Notas Mus. La Plata, Bot.
15: 37-48.
Cabrera AL. 1951. Notas sobre compuestas de
la America Austral. – Darwiniana 9: 363-386.
Cabrera AL. 1954a. Compuèstas sudamericanas
nuevas o críticas II. – Notas Mus., Univ. Nac. Eva Peron 17, 84: 71-80.
Cabrera AL. 1954b. Las especies del género
’Nardophyllum’. – Notas Mus. La Plata, Bot. 17: 55-66.
Cabrera AL. 1955. Un nuevo género de
Mutíseas del Peru. – Bol. Soc. Argentina Bot. 6: 40-44.
Cabrera AL. 1957. El género Senecio
(Comositae) en Brasil, Paraguay y Uruguay. – Arch. Jard. Bot. Rio de Janeiro
15: 161-325.
Cabrera AL. 1958. El género Belloa
Rémy. – Bol. Soc. Argent. Bot. 7: 79-85.
Cabrera AL. 1959. Revisión del género
Dasyphyllum (Compositae). – Rev. Mus. La Plata, Bot. 9: 21-100.
Cabrera AL. 1961a. Compuestas Argentinas.
Clave para la determinación de los géneras. – Rev. Mus. Argent. Cienc. Nat.
Bernardino Revadaira e Inst. Nacc. Invest. Cienc. Nat., Bot. 2: 291-362.
Cabrera AL. 1961b. Observaciones sobre las
Inuleae-Gnaphalineae (Compositae) de América del Sur. – Bol. Soc. Argent.
Bot. 9: 359-386.
Cabrera AL. 1963. Estudios sobre el género
Hypochoeris. – Bol. Soc. Argent. Bot. 10: 166-195.
Cabrera AL. 1965. Revisión del género
Mutisia (Compositae). – Opera Lilloana 13: 1-227.
Cabrera AL. 1966. The genus
Lagenophora (Compositae). – Blumea 14: 285-308.
Cabrera Al. 1969a. El género
Moquinia (Compositae). – Bol. Soc. Argentina Bot. 11: 255-261.
Cabrera AL. 1969b. Compuestas nuevas de
Patagonia. – Bol. Soc. Argentina Bot. 11: 271-275.
Cabrera AL. 1970. Actinoseris, nuevo
género de compuestas. – Bol. Soc. Argentina Bot. 13: 45-52.
Cabrera AL. 1971. Revisión del género
Gochnatia (Compositae). – Rev. Mus. La Plata, Bot., n. s., 12:
1-160.
Cabrera AL. 1974. Tres Compositae nuevas de
Minas Gerais (Brasil). – Bol. Mus. Bot. Munic. 15: 1-4.
Cabrera AL. 1977. Mutisieae – systematic
review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae 2, Academic Press, London, pp. 1039-1066.
Cabrera AL. 1982. Revisión del género
Nassauvia (Campositae). – Darwiniana 24: 283-379.
Cabrera AL. 1985. El género Senecio
(Compositae) en Bolivia. – Darwiniana 26: 79-217.
Cabrera AL, Zardini EM. 1980. Sinopsis
preliminar de las especies Argentinas del género Senecio
(Compositae). – Darwiniana 22: 427-492.
Cabrera R L. 2001. Six new species of
Acourtia (Asteraceae) and
a historical account of Acourtia mexicana. – Brittonia 53:
416-429.
Calabria LM, Emerenciano VP, Scotti MT, Mabry
TJ. 2007. Phylogenetic analysis of the tribes of the Asteraceae based on phytochemical
data. – Nat. Prod. Comm. 3: 277-285.
Calabria LM, Emerenciano VP, Scotti MT, Mabry
TJ. 2009. Secondary chemistry of Compositae. – In: Funk VA, Susanna A,
Stuessy TF, Bayer RJ (eds), Systematics, evolution, and biogeography of
Compositae, International Association for Plant Taxonomists, Wien, pp.
73-88.
Calderon JS, Quijano L, Cristia M, Gomez K,
Rios T. 1983. Labdane diterpenes from Brickellia veronicaefolia. –
Phytochemistry 22: 1783-1785.
Calleja JA, Garcia-Jacas N, Roquet C, Susanna
A. 2016. Beyond the Rand Flora pattern: phylogeny and biogeographical history
of Volutaria (Compositae). – Taxon 65: 315-332.
Callmander MW, Phillipson PB. 2012. The genus
Vernoniopsis Humbert (Asteraceae) in Madagascar. –
Candollea 66: 409-412.
Calvo J, Roque N. 2018. Taxonomic revision of
the Neotropical genus Campuloclinium (Eupatorieae, Compositae). –
Syst. Bot. 43: 602-627.
Calvo J, Álvarez I, Aedo C, Pelser PB. 2013.
A phylogenetic analysis and new delimitation of Senecio sect.
Crociseris (Compositae: Senecioneae), with evidence of intergeneric
hybridization. – Taxon 62: 127-140.
Campagna ML, Downie SR. 1998. The intron in
chloroplast gene rpl16 is missing from the flowering plant families Geraniaceae, Goodeniaceae and Plumbaginaceae.
– Trans. Illinois State Acad. Sci. 9: 1-11.
Canne JM. 1977. A revision of the genus
Galinsoga (Compositae: Heliantheae). – Rhodora 79: 319-389.
Canne-Hilliker JM. 1992. An emended
description, chromosome counts, and a key to South American Galinsoga
(Asteraceae, Heliantheae). –
Taxon 41: 661-666.
Cano-Maqueda J, Talavera S, Arista M,
Catalán P. 2008. Speciation and biogeographical history of the Campanula
lusitanica complex (Campanulaceae) in the Western
Mediterranean region. – Taxon 57: 1252-1266.
Capasso A, Urruñaga R, Garofala L,
Sorrentino L, Aquino R. 1996. Phytochemical and pharmacological studies on the
medical herb Acicarpha tribuloides. – Intern. J. Pharmacognosy 34:
255-261.
Carano E. 1915. Ricerche sull’embriogenesi
delle Asteraceae. – Ann. Bot.
(Roma) 13: 251-301.
Cariaga KA, Pruski JF, Oviedo R, Anderberg
AA, Lewis CE, Francisco-Ortega J. 2008. Phylogeny and systematic position of
Feddea (Asteraceae:
Feddeeae): a taxonomically enigmatic and critically endangered genus endemic to
Cuba. – Syst. Bot. 33: 193-202.
Carlquist SJ. 1956a. On the generic limits of
Eriophyllum (Compositae) and related genera. – Madroño 13:
226-239.
Carlquist SJ. 1956b. On the occurrence of
intercellular pectic warts in Compositae. – Amer. J. Bot. 43: 425-429.
Carlquist SJ. 1957a. Leaf anatomy and
ontogeny in Argyroxiphium and Wilkesia (Compositae). –
Amer. J. Bot. 44: 696-705.
Carlquist SJ. 1957b. The genus
Fitchia (Compositae). – Univ. Calif. Publ. Bot. 29: 1-144.
Carlquist SJ. 1957c. Anatomy of Guayana
Mutisieae. – Mem. New York Bot. Gard. 9: 441-476.
Carlquist SJ. 1957d. Wood anatomy of
Mutisieae (Compositae). – Trop. Woods 106: 29-45.
Carlquist SJ. 1957e. Systematic anatomy of
Hesperomannia. – Pacific Sci. 11: 207-215.
Carlquist SJ. 1958a. Anatomy of Guayana
Mutisieae II. – Mem. New York Bot. Gard. 10: 157-184.
Carlquist SJ. 1958b. Wood anatomy of
Heliantheae (Compositae). – Trop. Woods 108: 1-30.
Carlquist SJ. 1958c. Structure and ontogeny
of glandular trichomes of Madiinae (Compositae). – Amer. J. Bot. 45:
675-682.
Carlquist SJ. 1958d. Anatomy and systematic
position of Centaurodendron and Yunquea (Compositae). –
Brittonia 10: 78-93.
Carlquist SJ. 1958e. The woods and flora of
the Florida Keys. Compositae. – Trop. Woods 109: 1-37.
Carlquist SJ. 1959a. Vegetative anatomy of
Dubautia, Argyroxiphium, and Wilkesia (Compositae).
– Pacific Sci. 13: 195-210.
Carlquist SJ. 1959b. Studies on Madiinae:
anatomy, cytology, and evolutionary relationships. – Aliso 4: 171-236.
Carlquist SJ. 1959c. Wood anatomy of
Helenieae (Compositae). – Trop. Woods 111: 19-39.
Carlquist SJ. 1959d. The leaf of
Calycadenia and its glandular appendages. – Amer. J. Bot. 46:
70-80.
Carlquist SJ. 1959e. Glandular structures of
Holocarpha and their ontogeny. – Amer. J. Bot. 46: 300-308.
Carlquist SJ. 1960a. Wood anatomy of
Cichorieae (Compositae). – Trop. Woods 112: 65-91.
Carlquist SJ. 1960b. Wood anatomy of Astereae
(Compositae). – Trop. Woods 113: 54-84.
Carlquist SJ. 1961. Wood anatomy of Inuleae
(Compositae). – Aliso 5: 21-37.
Carlquist SJ. 1962a. Wood anatomy of
Senecioneae (Compositae). – Aliso 5: 123-146.
Carlquist SJ. 1962b. Ontogeny and comparative
anatomy of thorns of Hawaiian Lobeliaceae. – Amer. J. Bot. 49: 413-419.
Carlquist SJ. 1962c. Trematolobelia:
seed dispersal; anatomy of fruit and seeds. – Pacific Sci. 16: 126-134.
Carlquist SJ. 1964. Wood anatomy of
Vernonieae (Compositae). – Aliso 5: 451-467.
Carlquist SJ. 1965a. Island Life: a natural
history of the islands of the world. – Natural History Press, Garden City,
New York.
Carlquist SJ. 1965b. Wood anatomy of Cynareae
(Compositae). – Aliso 6: 13-24.
Carlquist SJ. 1965c. Wood anatomy of
Eupatorieae (Compositae). – Aliso 6: 89-103.
Carlquist SJ. 1966a. Wood anatomy of
Anthemideae, Ambrosieae, Calenduleae, and Arctotideae (Compositae). – Aliso
6: 1-23.
Carlquist SJ. 1966b. Wood anatomy of
Compositae: a summary, with comments on factors controlling wood evolution. –
Aliso 6: 25-44.
Carlquist SJ. 1966c. The biota of
long-distance dispersal II. Loss of dispersibility in Pacific Compositae. –
Evolution 20: 30-48.
Carlquist SJ. 1967. Anatomy and systematics
of Dendroseris (senso lato). – Brittonia 19: 99-121.
Carlquist SJ. 1969a. Wood anatomy of
Lobelioideae (Campanulaceae).
– Biotropica 1: 47-72.
Carlquist SJ. 1969b. Studies in Stylidiaceae: new taxa, field
observations, evolutionary tendencies. – Aliso 7: 13-64.
Carlquist SJ. 1969c. Wood anatomy of Goodeniaceae and the problem of
insular woodiness. – Ann. Missouri Bot. Gard. 56: 358-390.
Carlquist SJ. 1976a. New species of
Stylidium, and notes on Stylidiaceae from Western
Australia. – Aliso 8: 447-463.
Carlquist SJ. 1976a. Tribal
interrelationships and phylogeny of the Asteraceae. – Aliso 8: 465-492.
Carlquist SJ. 1978. New species of
Stylidium, with comments on evolutionary patterns in tropical Stylidiaceae. – Aliso 9:
308-322.
Carlquist SJ. 1979. Stylidium in
Arnhem Land: new species, modes of speciation on the sandstone plateau, and
comments on floral mimicry. – Aliso 9: 411-461.
Carlquist SJ. 1981a. Types of cambial
activity and wood anatomy of Stylidium (Stylidiaceae). – Amer. J. Bot.
68: 778-785.
Carlquist SJ. 1981b. Studies in Stylidiaceae: monocotyly in the
family; nomenclatural change. – Aliso 10: 35-38.
Carlquist SJ. 1982. Wood and bark anatomy of
Scalesia (Asteraceae).
– Aliso 10: 301-312.
Carlquist SJ. 1983a. Observations on the
vegetative anatomy of Crepidiastrum and Dendrocacalia (Asteraceae). – Aliso 10:
383-395.
Carlquist SJ. 1983b. Wood anatomy of Calyceraceae and Valerianaceae, with
comments on aberrant perforation plates in predominantly herbaceous groups of
dicotyledons. – Aliso 10: 413-425.
Carlquist SJ. 1995. A fully fertile
intergeneric hybrid derivative from Argyroxiphium sandwicense ssp.
macrocephalum x Dubautia menziesii (Asteraceae) and its relevance to
plant evolution in the Hawaiian Islands. – Amer. J. Bot. 82: 1574-1581.
Carlquist SJ. 1997a. Pentaphragma: a
unique wood and its significance. – IAWA J. 18: 3-12.
Carlquist SJ. 1997b. Wood anatomy of
Argyroxiphium (Asteraceae): adaptive radiation and
ecological correlations. – J. Torrey Bot. Soc. 124: 1-10.
Carlquist SJ. 1998a. Wood anatomy of
Wilkesia (Asteraceae)
with relation to systematics, organography, and habit. – J. Torrey Bot. Soc.
125: 261-267.
Carlquist SJ. 1998b. Wood anatomy of
Dubautia (Asteraceae:
Madiinae) in relation to adaptive radiation. – Pac. Sci. 52: 356-368.
Carlquist SJ. 2001. Wood anatomy of the
endemic woody Asteraceae of St.
Helena I: phyletic and ecological aspects. – Bot. J. Linn. Soc. 137:
197-210.
Carlquist SJ, DeVore ML. 1998. Wood anatomy
of Calyceraceae with reference
to ecology, habit, and systematic relationships. – Aliso 17: 63-76.
Carlquist SJ, Eckhart VM. 1982. Wood anatomy
of Darwiniothamnus, Lecocarpus, and Macraea (Asteraceae). – Aliso 10:
291-300.
Carlquist SJ, Grant ML. 1963. Studies in
Fitchia (Compositae): novelties from the Society Islands; anatomical
studies. – Pacific Sci. 17: 282-298.
Carlquist SJ, Lowrey TK. 2003. Wood anatomy
of Hawaiian and New Guinean species of Tetramolopium (Asteraceae): ecological and
systematic aspects. – Pacific Sci. 57: 171-179.
Carlquist SJ, Lowrie A. 1989. Two new species
of Stylidium from Western Australia. – Phytologia 67: 368-376.
Carlquist SJ, MacLachlan AA. 1992.
Nonglandular trichomes of Californian and Hawaiian tarweeds: surface
ultrastructure and its significance. – Aliso 13: 487-498.
Carlquist SJ, Baldwin BG, Carr GD (eds).
2004. Tarweeds and silverswords: evolution of the Madiinae (Asteraceae). – Missouri Botanical
Garden, St. Louis, Missouri.
Carlström A. 1986. A revision of the
Campanula drabifolia complex (Campanulaceae). – Willdenowia
15: 375-387.
Carolin RC. 1959. Floral structure and
anatomy in the family Goodeniaceae Dumort. – Proc.
Linn. Soc. New South Wales 84: 242-255.
Carolin RC. 1960a. Floral structure and
anatomy in the family Stylidiaceae Swartz. – Proc.
Linn. Soc. New South Wales 85: 189-196.
Carolin RC. 1960b. The structures involved in
the presentation of pollen to visiting insects in the order Campan[ul]ales. –
Proc. Linn. Soc. New South Wales 85: 197-205.
Carolin RC. 1966. Seeds and fruit of the Goodeniaceae. – Proc. Linn. Soc.
New South Wales 91: 58-83.
Carolin RC. 1967a. The concept of the
inflorescence in the order Campanulales. – Proc. Linn. Soc.
New South Wales 92: 7-26.
Carolin RC. 1967b. The genus Velleia
Sm. – Proc. Linn. Soc. New South Wales 92: 27-57.
Carolin RC. 1967c. Coopernookia: a
new genus of Goodeniaceae. –
Proc. Linn. Soc. New South Wales 92: 209-216.
Carolin RC. 1971. The trichomes of the Goodeniaceae. – Proc. Linn. Soc.
New South Wales 96: 8-22.
Carolin RC. 1974. Nigromnia, a new
genus of Goodeniaceae. –
Nuytsia 1: 292-293.
Carolin RC. 1978. The systematic
relationships of Brunonia. – Brunonia 1: 9-29.
Carolin RC. 1979. The genus Calogyne
R. Br. in Australia. – Brunonia 2: 1-17.
Carolin RC. 1980a. Pattern of the seed
surface of Goodenia and related genera. – Aust. J. Bot. 28:
123-137.
Carolin RC. 1980b. New species and new
combinations in Goodeniaceae and
Campanulaceae. – Telopea 2:
63-75.
Carolin RC. 1990a. Nomenclatural notes, new
taxa and the systematic arrangement in the genus Scaevola (Goodeniaceae) including synonyms.
– Telopea 3: 477-515.
Carolin RC. 1990b. Nomenclatural notes and
new taxa in the genus Goodenia (Goodeniaceae). – Telopea 3:
517-570.
Carolin RC. 1992. Brunoniaceae. – In:
George AS (ed), Flora of Australia 35, Australian Government Publ. Service,
Canberra, pp. 1-3.
Carolin RC. 2006 [2007]. Goodeniaceae. – In: Kubitzki K,
Kadereit JW, Jeffrey C (eds), The families and genera of vascular plants VIII.
Flowering plants. Eudicots. Asterales, Springer, Berlin, Heidelberg, New York,
pp. 589-598.
Carolin RC, Kadereit JW, Lundberg J. 2006
[2007]. Stylidiaceae. – In:
Kubitzki K, Kadereit JW, Jeffrey C (eds), The families and genera of vascular
plants VIII. Flowering plants. Eudicots. Asterales, Springer, Berlin,
Heidelberg, New York, pp. 614-619.
Carolin RC, Rajput MTM, Morrison D. 1992. Goodeniaceae. – In: George AS
(ed), Flora of Australia 35, Australian Government Publ. Service, Canberra, pp.
4-300.
Carr GD. 1975. Chromosome evolution and
aneuploid reduction in Calycadenia pauciflora (Asteraceae). – Evolution 29:
681-699.
Carr GD. 1977. A cytologial conspectus of the
genus Calycadenia (Asteraceae): an example of
contrasting modes of evolution. – Amer. J. Bot. 64: 694-703.
Carr GD. 1985a. Monograph of the Hawaiian
Madiinae (Asteraceae):
Argyroxiphium, Dubautia, and Wilkesia. –
Allertonia 4: 1-123.
Carr GD. 1985b. Habital variation in the
Hawaiian Madiinae (Heliantheae) and its relevance to generic concepts in the
Compositae. – Taxon 34: 22-25.
Carr GD. 1987. Beggar’s ticks and tarweeds:
masters of adaptive radiation. – Trends Ecol. Evol. 2: 192-195.
Carr GD. 2003a. Chromosome evolution in
Madiinae. – In: Carlquist S, Baldwin BG, Carr GD (eds), Tarweeds &
silverswords: evolution of the Madiinae (Asteraceae), Missouri Botanical
Garden Press, St. Louis, Missouri, pp. 53-78.
Carr GD. 2003b. Hybridization in Madiinae.
– In: Carlquist S, Baldwin BG, Carr GD (eds), Tarweeds & silverswords:
evolution of the Madiinae (Asteraceae), Missouri Botanical
Garden Press, St. Louis, Missouri, pp. 79-104.
Carr GD, Kyhos DW. 1981. Adaptive radiation
in the Hawaiian silversword alliance (Compositae-Madiinae) I. Cytogenetics of
spontaneous hybrids. – Evolution 35: 543-556.
Carr GD, Kyhos DW. 1986. Adaptive radiation
of the Hawaiian silversword alliance (Compositae-Madiinae) II. Cytogenetics of
artificial and natural hybrids. – Evolution 40: 959-976.
Carr GD, Powell EA, Kyhos DW. 1986.
Self-incompatibility in the Hawaiian Madiinae (Compositae): an exception to
Baker’s rule. – Evolution 40: 430-436.
Carr GD, Robichaux R, Witter MS, Kyhos DW.
1989. Adaptive radiation of the Hawaiian silversword alliance
(Compositae-Madiinae): a comparison with the Hawaiian picture-winged
Drosophila. – In: Giddings LV, Kaneshiro KY, Anderson WW (eds),
Genetics, speciation, and the founder principle, Oxford University Press,
Oxford, New York, pp. 79-97.
Carr GD, Baldwin BG, Kyhos DW. 1996.
Cytogenetic implications of artificial hybrids between the Hawaiian silversword
alliance and North American tarweeds (Asteraceae: Heliantheae-Madiinae).
– Amer. J. Bot. 83: 653-660.
Carr GD, King RM, Powell M, Robinson H. 1999.
Chromosome numbers in Compositae XVIII. – Amer. J. Bot. 86: 1003-1013.
Carr RL, Carr GD. 1983. Chromosome races and
structural heterozygosity in Calycadenia ciliosa Greene (Asteraceae). – Amer. J. Bot. 70:
744-755.
Carrijo T, Garbin M, Picanço Leite W,
Mendonça C, Esteves R, Gonçalves-Esteves V. 2013. Pollen morphology of some
related genera of Vernonieae (Asteraceae) and its taxonomic
significance. – Plant Syst. Evol. 299: 1275-1283.
Carrillo-Reyes P. 2008. A new species of
Perityle (Asteraceae,
Perityleae) from western Mexico. – Syst. Bot. 33: 459-461.
Carter CR. 178. The cytology of
Brachycome II. The subgenus Metabrachycome: a general survey.
– Aust. J. Bot. 26: 699-706.
Cave RL, Birch CJ, Hammer GL, Erwin JE,
Johnston ME. 2010. Floral ontogeny in Brunonia australis (Goodeniaceae) and Calandrinia
speciosa (Portulacaceae).
– Aust. J. Bot. 58: 61-69.
Cellinese N, Smith S, Edwards E, Kim S-T,
Haberle RC, Avramakis M, Donoghue MJ. 2009. Historical biogeography of the
endemic Campanulaceae of Crete.
– J. Biogeogr. 36: 1253-1269.
Cerana MM, Ariza Espinar L. 1995. Sobre la
presencia de domacios en Mikania (Asteraceae). – Kurtziana 24:
7-18.
Cerbah M, Coulaud J, Godelle B,
Šiljac-Yakovlev S. 1995. Genome size, fluorochrome banding, and karyotype
evolution in some Hypochoeris species. – Genome 38: 689-695.
Cerbah M, Souza-Chies T, Jubier MF, Lejeune
B, Siljak-Yakovlev S. 1998. Molecular phylogeny of the genus
Hypochaeris using internal transcribed spacers of nuclear rDNA:
inference for chromosomal evolution. – Mol. Biol. Evol. 15: 345-354.
Çeter T, Pinar N, İnceer H,
Hayirlioğlu-Ayaz S, Yaprak A. 2013. The comparative pollen morphology of
genera Matricaria L. and Tripleurospermum Sch. Bip. (Asteraceae) in Turkey. – Plant
Syst. Evol. 299: 959-977.
Chambers KL. 1955. A biosystematic study of
the annual species of Microseris. – Contr. Dudly Herb. (Stanford) 4:
207-312.
Chambers KL. 1963. Amphitropical species
pairs in Microseris and Agoseris (Compositae: Cichorieae).
– Quart. Rev. Biol. 38: 124-140.
Chan R K-G. 2000. Molecular systematics of
the goldfield genus Lasthenia (Compositae: Heliantheae sensu lato).
– Ph.D. diss., University of California, Berkeley, California.
Chandjian NS. 1990. Genus Anthemis
L. (Asteraceae) in Caucaso
meridionali. – Nov. Sist. Vyssh. Rast. 27: 152-163. [In Russian]
Chandler B. 1911. Note on Donatia
novae-zelandiae Hook. f. – Notes Roy. Bot. Gard. Edinb. 6: 43-48.
Chandler JM, Jan C-C, Beard BH. 1986.
Chromosomal differentiation among the annual Helianthus species. –
Syst. Bot. 11: 354-371.
Changzeng W, Dequan Y. 1997. Diterpenoid,
sesquiterpenoid and secoiridoid glucosides from Aster auriculatus. –
Phytochemistry 45: 1483-1487.
Chapman JL. 1966. Comparative palynology in
Campanulaceae. – Trans.
Kansas Acad. Sci. 69: 197-200.
Chapman MA, Tang S, Draeger D, Nambeesan S,
Shaffer H, Barb, JG, Knapp SJ, Burke JM. 2012. Genetic analysis of floral
symmetry in Van Gogh’s sunflowers reveals independent recruitment of
CYCLOIDEA genes in the Asteraceae.
– PloS Genet. 8, e1002628.
Chebataroff PJ. 1981. Una nueva especie del
género Sommerfeltia (Compositae). – Hickenia 1: 233-235.
Chen L-Y, Wang Q-F, Renner SS. 2016. East
Asian Lobelioideae and ancient divergence of a giant rosett Lobelia in
Himalayan Bhutan. – Taxon 65: 293-304.
Chen Y-L. 1984. Two new species and a new
name in Chinese Compositae. – Kew Bull. 39: 157-161.
Cheon K-S, Yoo K-O. 2013. Phylogeny of
Hanabusaya (Campanulaceae), a Korean endemic,
based on ITS sequences of nuclear ribosomal DNA. – J. Syst. Evol. 51:
704-714.
Cherneva OV. 1983. The genus
Hyacanthium (Asteraceae)
and its representatives in middle Asia. – Bot. Žurn. 68: 632-635. [In
Russian]
Cherneva OV. 1988. New supraspecific taxa of
the genus Cousinia (Asteraceae). – Bot. Žurn. 73:
594-597. [In Russian]
Cherneva OV. 1990. Problems of evolution of
the genus Cousinia (Asteraceae). – Bot. Žurn. 75:
811-815. [In Russian]
Chiappini M. 1955. Ricerche sullo sviluppo
embriologico di alcune specie del genere Centaurea L. (Asteraceae). – Nuovo Giorn. Bot.
Ital., n. s., 61: 274-289.
Chiarini FE, Barboza GE, Cantero JJ. 2015.
The chromosomes of the rare and endemic genus Famatinanthus
(Famatinanthoideae, Asteraceae).
– Arnaldoa 22: 495-506.
Chmielewski JG. 1997. A taxonomic revision of
the Antennaria media (Asteraceae: Inuleae) polyploid
species complex in western North America. – Brittonia 49: 309-327.
Chmielewski JG, Chinnappa CC. 1987. The genus
Antennaria (Asteraceae:
Inuleae) in North America: multivariate analysis of variation patterns in
Antennaria rosea sensu lato. – Can. J. Bot. 66: 1583-1609.
Cho, M.S., Kim, S.-H., Yang, J. Y., Crawford, D. J., Stuessy, T. F.,
López-Sepúlveda, P., Kim, S.-C. 2020. Molecular evolution of an endemic lineage from
the Juan Fernández Islands. – Front. Plant Sci.,
05 November 2020. https://doi.org/10.3389/fpls.2020.594272
Choudry SA, Tanaka R, Taniguchi K, Oginuma K.
1980. Karyomorphology of Senecio nikoensis. – Chromosome Inform.
Serv. 29: 8-10.
Chouksanova NA, Sveshnikova LI, Alexandrova
TV. 1968a. Data on karyology of the family Compositae Giseke. – Citologija
10: 198-206. [In Russian]
Chouksanova NA, Sveshnikova LI, Alexandrova
TV. 1968b. A new evidence on chromosome numbers in species of the family
Compositae Giseke. – Citologija 10: 381-386. [In Russian]
Christensen LP. 1992. Acetylenes and related
compounds in Anthemideae. – Phytochemistry 31: 7-49.
Christin P-A, Osborne CP, Sage RF, Arakaki M,
Edwards EJ. 2011. C4 eudicots are not younger than C4
monocots. – J. Experim. Bot. 62: 3171-3181.
Christoff M, Popoff A. 1933. Cytologische
Studien über die Gattung Hieracium. – Planta 20: 340-447.
Chuang TI, Ornduff R. 1992. Seed morphology
and systematics of Menyanthaceae. – Amer. J. Bot.
79: 1396-1406.
Chung I. 1965. Revision of
Barnadesia. – Taesutang Press, Seoul, Korea.
Clark C, Sanders DL. 1986. Floral ultraviolet
patterns in the Encelia alliance (Asteraceae: Heliantheae). –
Madroño 33: 130-135.
Clark WD. 1979. The taxonomy of
Hazardia (Compositae: Astereae). – Madroño 26: 105-127.
Clarke CB. 1876. Compositae indicae. –
Calcutta.
Claßen-Bockhoff R. 1992. Florale
Differenzierung in komplex organisierten Asteraceen-Köpfchen. – Flora 186:
1-22.
Claßen-Bockhoff R. 1996. Functional units
beyond the level of the capitulum and cypsela in Compositae. – In: Caligari
P, Hind DJN (eds), Compositae: biology and utilization, Proceedings of the
International Compositae Conference, Kew, 1994, vol. 2, Royal Botanic Gardens,
Kew, pp. 129-160.
Claßen-Bockhoff R, Froebe H, Langerheins D.
1989. Die Infloreszenzstruktur von Gundelia tournefortii L. (Asteraceae). – Flora 182:
463-479.
Clayton JS, Tanner CC. 1985. Nymphoides
geminata (R. Br.) Kuntze in New Zealand. – New Zealand J. Bot. 23:
187-190.
Clevinger JA, Panero JL. 2000. Phylogenetic
analysis of Silphium and subtribe Engelmanniinae (Asteraceae: Heliantheae) based on ITS
and ETS sequence data. – Amer. J. Bot. 87: 565-572.
Coates DJ. 1979a. Karyotype analysis in
Stylidium crossocephalum (Angiospermae: Stylidiaceae). – Chromosoma 72:
347-356.
Coates DJ. 1979b. Cytoevolution and
speciation in the scale leaved triggerplants (Stylidium section
Squamosae). – Ph.D. diss., University of Western Australia,
Nedlands, Australia.
Coates DJ. 1981. Chromosome, morphometric and
breeding system studies in the Stylidium caricifolium species complex
(Stylidiaceae). – Aust. J.
Bot. 29: 397-417.
Coates DJ. 1982. Chromosome variation and
species relationships in the scale-leaved tiggerplants (Stylidium
section Squamosae). – Aust. J. Bot. 30: 121-130.
Coates DJ, James SH. 1979. Chromosome
variation in Stylidium crossocephalum (Angiospermae: Stylidiaceae) and the dynamic
co-adaptation of its lethal system. – Chromosoma 72: 357-376.
Coates DJ, Carstairs S, Hamley VL. 2003.
Evolutionary patterns and genetic structure in localized and widespread species
in the Stylidium caricifolium complex (Stylidiaceae). – Amer. J. Bot.
90: 997-1008.
Col A. 1899-1901. Quelques recherches sur
l’appareil sécréteur des Composées. – J. Bot. (Morot) 13: 234-252; 15:
166-168.
Col A. 1903-1904. Recherches sur l’appareil
sécréteur interne des Composées. – J. Bot. (Morot) 17: 252-318; 18:
110-133, 153-175.
Coleman JR. 1968a. A cytotaxonomic study in
Verbesina (Compositae). – Rhodora 70: 95-102.
Coleman JR. 1968b. Chromosome numbers in some
Brazilian Compositae. – Rhodora 70: 228-240.
Coleman JR. 1970. Additional chromosome
numbers in Brazilian Compositae – Rhodora 72: 94-99.
Coleman M, Forbes DG, Abbott RJ. 2001. A new
subspecies of Senecio mohavensis (Compositae) reveals Old-New World
species disjunction. – Edinburgh J. Bot. 58: 389-403.
Coleman M, Liston A, Kaderit JW, Abbott RJ.
2003. Repeat intercontinental dispersal and Pleistocene speciation in disjunct
Mediterranean and desert Senecio (Asteraceae). – Amer. J. Bot 90:
1446-1454.
Colozza A. 1907. Studio anatomico sulle Goodeniaceae. – Nuovo Giorn. Bot.
Ital. 14: 304-326.
Colozza A. 1908. Studio anatomico sulle Goodeniaceae. – Nuovo Giorn. Bot.
Ital. 15: 5-92.
Comes HP, Abbott RJ. 2001. Molecular
phylogeography, reticulation, and lineage sorting in Mediterranean
Senecio sect. Senecio (Asteraceae). – Evolution 55:
1943-1962.
Compton RH. 1935. The genus
Peyrousea DC. – J. South Afr. Bot. 1: 71-74.
Constance L. 1937. A systematic study of the
genus Eriophyllum Lag. – Univ. Calif. Publ. Bot. 18: 69-135.
Constantinidis T, Kalpoutzakis E. 2005. A new
species of Achillea (Asteraceae: Anthemideae) from
South-East Peloponnisos, Greece. – Bot. J. Linn. Soc. 147: 249-256.
Contandriopoulos J. 1972. Contribution à
l’étude cytotaxonomique des Campanulacées du Proche-Orent III. – Bull.
Soc. Bot. France 119: 75-94.
Contandriopoulos J. 1984. Differentiation and
evolution of the genus Campanula in the Mediterranean region. – In:
Grant WF (ed), Plant biosystematics, Academic Press, Toronto, pp. 141-158.
Contandriopoulos J, Quézel P, Pamukçuoğlu
A. 1971. Campanulacées nouvelles du pourtour méditerranéen oriental. –
Ann. Univ. Provence, Sci. 46: 53-61.
Cook CDK. 1990. Seed dispersal of
Nymphoides peltata (S. G. Gmelin) O. Kuntze (Menyanthaceae). – Aquatic Bot.
37: 325-340.
Cooper DC, Mahony KL. 1935. Cytological
observations on certain Compositae. – Amer. J. Bot. 22: 843-848.
Cooper GO. 1942. Microsporogenesis and
development of seed in Lobelia cardinalis. – Bot. Gaz. 104:
72-81.
Correa A. 2003. Revision of the genus
Paragynoxys (Asteraceae,
Senecioneae-Tussilagininae). – Brittonia 55: 157-168.
Cosner ME. 1993. Phylogenetic and molecular
evolutionary studies of chloroplast DNA variation in the Campanulaceae. – Ph.D. diss.,
The Ohio State University, Columbus, Ohio.
Cosner ME, Crawford DJ. 1990. Allozyme
variation in Coreopsis Sect. Coreopsis (Compositae). –
Syst. Bot. 15: 256-265.
Cosner ME, Jansen RK, Lammers TG. 1994.
Phylogenetic relationships in the Campanulales based on rbcL
sequences. – Plant Syst. Evol. 190: 79-95.
Cosner ME, Jansen RK, Palmer JD, Downie SR.
1997. The highly rearranged chloroplast genome of Trachelium caeruleum
(Campanulaceae): multiple
inversions, inverted repeat expansion and contraction, insertions/deletions,
and several repeat families. – Curr. Genet. 31: 419-429.
Cosner ME, Raubeson LA, Jansen RK. 2004.
Chloroplast DNA rearrangements in Campanulaceae: phylogenetic
utility of highly rearranged genomes. – BMC Evol. Biol. 4: 1-17.
Cox PB, Urbatsch LE. 1990. A phylogenetic
analysis of the coneflower genera (Asteraceae: Heliantheae). – Syst.
Bot. 15: 394-402.
Crawford DJ, Stuessy TF. 1981. The taxonomic
significance of anthochlors in the subtribe Coreopsidinae (Compositae,
Heliantheae). – Amer. J. Bot. 18: 107-117.
Crawford DJ, Stuessy TF, Silva OM. 1987.
Allozyme divergence and the evolution of Dendroseris (Compositae:
Lactuceae) on the Juan Fernandez Islands. – Syst. Bot. 12: 435-443.
Crawford DJ, Whitkus R, Stuessy TF. 1987.
Plant evolution and speciation on oceanic islands. – In: Urbanska KM (ed),
Differentiation patterns in higher plants, Academic Press, London, pp.
183-199.
Crawford DJ, Palmer JD, Kobayashi M. 1990.
Chloroplast DNA restriction site variation and the phylogeny of
Coreopsis section Coreopsis (Asteraceae). – Amer. J.Bot. 77:
552-558.
Crawford DJ, Stuessy TF, Lammers TG, Silva M.
1990. Allozyme variation and evolutionary relationships among three species of
Wahlenbergia (Campanulaceae) in the Juan
Fernandez Islands. – Bot. Gaz. 151: 119-124.
Crawford DJ, Palmer JD, Kobayashi M. 1991.
Chloroplast DNA restriction site variation, phylogenetic relationships, and
character evolution among sections of North American Coreopsis (Asteraceae). – Syst. Bot. 16:
211-224.
Crawford DJ, Palmer JD, Kobayashi M. 1992.
Chloroplast DNA restriction site variation and the evolution of the annual
habit in North American Coreopsis (Asteraceae). – In: Soltis PS,
Soltis DE, Doyle JJ (eds), Molecular systematics of plants, Chapman and Hall,
New York, pp. 280-294.
Crawford DJ, Whitkus R, Haines DW, Cosner MB,
Silva OM, Lopez P. 1992. Allozyme diversity within and divergence among four
species of Robinsonia (Asteraceae: Senecioneae), a genus
endemic to the Juan Fernandez Islands, Chile. – Amer. J. Bot. 79: 962-966.
Crawford DJ, Stuessy TF, Cosner MB, Haines
DW, Silva O M, Baeza M. 1992. Evolution of the genus Dendroseris (Asteraceae: Lactuceae) on the Juan
Fernandez Islands: evidence from chloroplast and ribosomal DNA. – Syst. Bot.
17: 676-682.
Crawford DJ, Stuessy TF, Cosner MB, Haines
DW, Silva O M. 1993. Ribosomal and chloroplast DNA restriction site mutations
and the radiation of Robinsonia (Asteraceae: Senecioneae) on the Juan
Fernandez Islands. – Plant Syst. Evol. 184: 233-239.
Crawford DJ, Kimball RT, Tadesse M. 2001. The
generic placement of a morphologically enigmatic species in Asteraceae: evidence from ITS
sequences. – Plant Syst. Evol. 228: 63-69.
Crawford DJ, Lowrey TK, Anderson GJ,
Bernardello G, Santos-Guerra A, Stuessy TF. 2009. Genetic diversity in Asteraceae endemic to oceanic
islands: Baker’s Law and polyploidy. – In: Funk VA, Susanna A, Stuessy TF,
Bayer RJ (eds), Systematics, evolution, and biogeography of Compositae,
International Association for Plant Taxonomists, Wien, pp. 139-151.
Crawford DJ, Tadesse M, Mort ME, Kimball RT,
Randle CP. 2009. Coreopsideae. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ
(eds), Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 713-730.
Crepet WL, Stuessy TF. 1978. A
reinvestigation of the fossil Viguiera cronquistii (Compositae). –
Brittonia 30: 483-491.
Crespo MB, Serra L, Juan A. 1998.
Solenopsis (Lobeliaceae): a genus endemic in the Mediterranean region.
– Plant Syst. Evol. 210: 211-229.
Crété R. 1956. Contribution à l’étude
de l’albumen et de l’embryon chez les Campanulacées et les Lobéliacées.
– Bull. Soc. Bot. France 103: 446-454.
Crins WJ, Bohm BA. 1990. Flavonoid diversity
in relation to systematics and evolution of the tarweeds. – Ann. Missouri
Bot. Gard. 77: 73-83.
Crins WJ, Bohm BA, Carr GD. 1988. Flavonoids
as indicators of hybridization in a mixed population of lava-colonizing
Hawaiian tarweeds (Asteraceae:
Heliantheae: Madiinae). – Syst. Bot. 13: 567-571.
Crisci JV. 1974a. Marticorenia: a
new genus of Mutisieae (Compositae). – J. Arnold Arbor. 55: 38-55.
Crisci JV. 1974b. A numerical-taxonomic study
of the subtribe Nassauviinae (Compositae, Mutisieae). – J. Arnold Arbor. 55:
568-610.
Crisci JV. 1976. Revisión del género
Leucheria (Compositae: Mutisieae). – Darwiniana 20: 9-126.
Crisci JV. 1980. Evolution in the subtribe
Nassauviinae (Compositae, Mutisieae): a phylogenetic reconstruction. – Taxon
29: 137-153.
Cristov M, Panayotov I. 1991. Hybrids between
the genera Helianthus and Tithonia and their study. – Helia
14: 24-34.
Cron GV, Balkwill K. 2006. Bolandia
(Asteraceae, Senecioneae): a new
genus endemic to southern Africa. – Novon 16: 224-230.
Cron GV, Nordenstam B. 2009. Senecio
umbricola (Asteraceae-Senecioneae), a new
species from the Cederberg, South Africa. – South Afr. J. Bot. 75:
246-248.
Cron GV, Balkwill K, Knox EB. 2006. Two new
species of Cineraria (Senecioneae, Asteraceae) from KwaZulu-Natal, South
Africa. – South Afr. J. Bot. 72: 34-39.
Cron GV, Balkwill K, Knox EB. 2008.
Phylogenetic evidence for the generic circumscription of Cineraria L.
(Asteraceae-Senecioneae). –
Taxon 57: 779-798.
Cronquist A. 1947. Revision of the North
American species of Erigeron North of Mexico. – Brittonia 6:
121-302.
Cronquist A. 1955. Phylogeny and taxonomy of
the Compositae. – Amer. Midl. Natur. 53: 478-511.
Cronquist A. 1977. The Compositae revisited.
– Brittonia 29: 137-153.
Cronquist A. 1985. History of generic
concepts in the Compositae. – Taxon 34: 6-10.
Cronquist A, Keck D. 1957. A reconstitution
of the genus Machaeranthera. – Brittonia 9: 231-239.
Cross EW, Quinn CJ, Wagstaff SJ. 2002.
Molecular evidence for the polyphyly of Olearia (Astereae: Asteraceae). – Plant Syst. Evol.
235: 99-120.
Crowl AA, Miles NW, Visger CJ, Hansen K,
Ayers T, Haberle R, Cellinese N. 2016. A global perspective on Campanulaceae: biogeographic,
genomic, and floral evolution. – Amer. J. Bot. 103: 233-245.
Cruz-Mazo G, Buide ML, Samuel R, Narbona E.
2009. Molecular phylogeny of Scorzoneroides (Asteraceae): evolution of heterocarpy
and annual habit in unpredictable environments. – Mol. Phylogen. Evol. 53:
835-847.
Csongor G. 1947. Monographie critique des
espèces du genre Leontodon dans les Bassins Carpathiques. – Acta
Geobot. Hung., s. n., 1: 51-69.
Cuatrecasas J. 1950. Studies on Andean
Compositae I. – Fieldiana Bot. 27(1): 1-54.
Cuatrecasas J. 1951. Studiess on Andean
Compositae II. – Fieldiana Bot. 27(2): 1-74.
Cuatrecasas J. 1954. Synopsis der Gattung
Loricaria Wedd. – Feddes Repert. Spec. Nov. Regni Veg. 56:
149-172.
Cuatrecasas J. 1954b. El género
Mniodes. – Folia Biol. Andina 1: 17.
Cuatrecasas J. 1969. Prima Flora Colombiana
3. Compositae-Astereae. – Webbia 24: 1-335.
Cuatrecasas J. 1970. Reinstatement of the
genus Llerasia (Compositae). – Biotropica 2: 39-45.
Cuatrecasas J. 1976. A new subtribe in the
Heliantheae (Compositae): Espeletiinae. – Phytologia 35: 42-61.
Cuatrecasas J. 1977. Westoniella, a
new genus of the Astereae from the Costa Rican páramos. – Phytologia 35:
471-487.
Cuatrecasas J. 1978. Studies in neotropical
Senecioneae, Compositae I. Reinstatement of genus Lasiocephalus. –
Phytologia 40: 307-312.
Cuatrecasas J. 1982. Miscellaneous notes on
neotropical flora XV. New taxa in the Astereae. – Phytologia 52: 166-177.
Cuatrecasas J. 2013. A systematic study of
the subtribe Espeletiinae (Heliantheae, Asteraceae). – Mem. New York Bot.
Gard. 107: 1-689.
Čuksanova NA, Svešnikova LI, Alexandrova
TV. 1968. Data on the karyology of the family Compositae Giseke. – Citologija
10: 198-206. [In Russian]
Culvenor CCJ, Edgar JA, Smith LW, Hirono I.
1976. The occurrence of senkirkine in Tussilago farfara. – Aust. J.
Chem. 29: 229-230.
Cunneen TM. 1995. Breeding for improvement of
the Marquerite Daisy (Argyranthemum sp.). – Acta Hort. 420:
101-103.
Cupido CN. 2003. Systematic studies in the
genus Merciera (Campanulaceae): a re-assessment of
species boundaries. – Adansonia, sér. III, 25: 33-44.
Cupido CN. 2006. A taxonomic revision of the
genus Merciera (Campanulaceae). – Bothalia 36:
1-11.
Cupido CN. 2009. Systematic studies of the
South African Campanulaceae
sensu stricto with an emphasis on generic delimitation. – Ph.D. diss.,
University of Cape Town, Cape Town.
Cupido CN. 2011. A new species of
Wahlenbergia from Western Cape, South Africa. – Bothalia 41:
178-181.
Cupido CN, Hedderson TAJ. 2007. Phylogenetic
re-assessment of generic boundaries in South African Campanulaceae with an emphasis on
Roella, Merciera and Prismatocarpus. – South Afr.
J. Bot. 73: 326.
Cupido CN, Weitz FM. 2016. Kericodon
(Campanulaceae s.s.), a new
monotypic wahlenbergioid genus from South Africa. – Kew Bull. 71: 56 DOI
10.1007/S12225-016-9671-4
Cupido CN, Eddie WMM, Tiedt LR. 2011.
Systematic and ecological significance of seed coat morphology in South African
Campanulaceae sensu stricto.
– Edinburg J. Bot. 68: 351-371.
Cupido CN, Prebble JM, Eddie WMM. 2013.
Phylogeny of southern African and Australasian wahlenbergioids (Campanulaceae) based on ITS and
trnL-F sequence data: implications for a reclassification. – Syst.
Bot. 38: 523-535.
Custrecasas J. 1989. Un género nuevo de
Astereae, Compositae, de Colombia. – An. Jard. Bot. Madrid 42: 415-426.
Czapik R. 1996. Problems of apomictic
reproduction in the families Compositae and Rosaceae. – Folia
Geobot. Phytotaxon. 3: 381-387.
Dagne K. 2001. Cytogenetics of new
Guizotia Cass. (Compositae) interspecific hybrids pertaining to
genomic and phylogenetic affinities. – Plant Syst. Evol. 230: 1-11.
Dahlgren KVO. 1915. Über die Embryologie von
Acicarpha tribuloides Juss. – Svensk Bot. Tidskr. 9: 184-191.
Dahlgren KVO. 1920. Zur Embryologie der
Kompositen mit besonderer Berücksichtigung der Endospermbildung. – Zeitschr.
Bot. 12: 481-516.
Dahlgren KVO. 1924. Studien über die
Endospermbildung der Kompositen. – Svensk Bot. Tidskr. 18: 177-203.
Damboldt J. 1965. Zytotaxonomische Revision
der isophyllen Campanulae in Europa. – Bot. Jahrb. Syst. 84:
302-358.
Damboldt J. 1968. Vorarbeiten zu einer
Revision der Gattung Asyneuma (Campanulaceae). – Willdenowia 5:
35-54.
Damboldt J. 1970. Revision der Gattung
Asyneuma. – Boissiera 17: 5-128.
Damboldt J. 1976. Materials for a flora of
Turkey 32: Campanulaceae. –
Notes Roy. Bot. Gard. Edinb. 35: 39-52.
Darnowski DW. 2002. Triggerplants. –
Rosenberg Publ., Australia.
Darnowski DW, Carroll DM, Plachno B, Kabanoff
E, Cinnamon E. 2006. Evidence of protocarnivory in triggerplants
(Stylidium spp.; Stylidiaceae). – Plant Biol. 8:
805-812.
Davies FG. 1978. Goodeniaceae. – In: Polhill RM
(ed), Flora of tropical East Africa, Crown Agents for Oversea Governments and
Administration, London, pp. 1-4.
Davies FG. 1981. The transfer of two
Carduncellus species to Atractylis (Compositae). – Kew
Bull. 36: 142.
Davis GL. 1948. Revision of the genus
Brachycome Cass. – Proc. Linn. Soc. New South Wales 73: 142-241.
Davis GL. 1952. Revision of the genus
Calotis R. Br. – Proc. Linn. Soc. New South Wales 77: 146-188.
Dawson MI, Breitwieser I, Ward JM. 1999.
Chromosome numbers in Craspedia, Ewartia and
Pterygopappus (Compositae: Gnaphalieae). – Aust Syst. Bot. 12:
671-674.
Deble LP, Marchiori JNC. 2007. Sinopse do
gênero Gamochaeta Weddell (Asteraceae-Gnaphalieae) no Brasil.
– Balduinia 10: 21-31.
Debourges D, Langlois N. 1982. Nouveaux
alcaloïdes du groupe de l’homoérythrinane isolés de Phelline
brachyphylla. – J. Nat. Prod. (Lloydia) 45: 163-167.
De Godoy SM, Da Silva JFM, De Paula GBN, Ruas
PM, Góes BD, De Fátima Ruas C. 2017. Phylogenetic relationships of Brazilian
Mikania species (Asteraceae, Eupatorieae) based on
multilocus DNA markers. – Bot. J. Linn. Soc. 184: 326-346.
DeJong DC. 1965. A systematic study of the
genus Astranthium (Compositae: Astereae). – Publ. Mus. Michigan
State Univ., Biol. Ser. 2: 429-528.
DeJong DC, Beaman JH. 1963. The genus
Olivaea (Compositae, Asteraceae). – Brittonia 15:
86-92.
DeJong DC, Longpre EK. 1963. Chromosome
studies in Mexican Compositae. – Rhodora 65: 225-240.
Dematteis M. 2002. Cytotaxonomic analysis of
South American species of Vernonia (Vernonieae: Asteraceae). – Bot. J. Linn. Soc.
139: 401-408.
Dematteis M. 2003. New species and new
combinations in Brazilian Vernonieae (Asteraceae). – Taxon 52:
281-286.
Dematteis M. 2006. Vernonanthura
warmingiana (Asteraceae:
Vernonieae), a new species from Brazil. – Brittonia 58: 182-188.
Dematteis M. 2007a. Taxonomic notes on genus
Chrysolaena (Vernonieae-Asteraceae), including a new species
endemic to Paraguay. – Ann. Bot. Fenn. 44: 56-64.
Dematteis M. 2007b. Vinicia
tomentosa, nuevo género y especie de Lychnophorinae (Vernonieae, Asteraceae) de Minas Gerais, Brsil.
– Bonplandia 16: 259-264.
Dematteis M, Fernández A. 1999. Chromosome
studies on the genus Isostigma (Heliantheae, Asteraceae). – Cytologia 64:
309-312.
Dematteis M, Pire SM. 2008. Pollen morphology
of some species of Vernonia s.l. (Vernonieae, Asteraceae) from Argentina and
Paraguay. – Grana 47: 117-129.
Dematteis M, Moler J, Angulo MB, Rovira AM.
2007. Chromosome studies on some Asteraceae from South America. –
Bot. J. Linn. Soc. 153: 221-230.
Demker O. 1998. Phylogeny and origin of the
bellflower family. – M.Sc. thesis, University of Uppsala, Sweden.
Denda T, Yokota M. 2003. Hybrid origins of
Ixeris nakazonei (Asteraceae, Lactuceae) in the Ryukyu
Archipelago, Japan: evidence from molecular data. – Bot. J. Linn. Soc. 141:
379-387.
Denham SS, Zavala-Gallo L, Pozner RE. 2014.
Morphology and taxonomic revision of Calycera. – Syst. Bot. 39:
1226-1249.
Denham SS, Zavala-Gallo L, Johnson LA, Pozner
RE. 2016. Insights into the phylogeny and evolutionary history of Calyceraceae. – Taxon 65:
1328-1344.
De Oliveira VM, Forni-Martins ER, Semir J.
2007. Cytotaxonomy of species of Vernonia, section
Lepidaploa, group Axilliflorae (Asteraceae, Vernonieae). – Bot. J.
Linn. Soc. 154: 99-108.
Desrochers AM, Bohm BA. 1995. Biosystematic
study of Lasthenia californica (Asteraceae). – Syst. Bot. 20:
65-84.
Desrochers AM, Dodge B. 2003. Phylogenetic
relationships in Lasthenia (Heliantheae: Asteraceae) based on nuclear rDNA
internal transcribed spacer (ITS) sequence data. – Syst. Bot. 28: 208-215.
Dethier D. 1982. Le genre Lactuca L.
(Asteraceae) en Afrique centrale.
– Bull. Jard. Bot. Natl. Belg. 52: 367-382.
Devesa JA. 1981. Contribución al studio
cariológico del género Carduus en la Península Ibérica. –
Lagascalia 10: 65-80.
Devesa JA, Talavera S. 1981a. Algunas
novedades taxonomicas y nomenclaturales para el género Carduus en la
Península Iberica. – Lagascalia 10: 59-63.
Devesa JA, Talavera S. 1981b. Revisión del
género Carduus (Compositae) en la Península Ibérica e Islas
Baleares. – Sevilla.
DeVore ML. 1991. The occurrence of
Acicarpha tribuloides (Calyceraceae) in eastern North
America. – Rhodora 93: 26-35.
DeVore ML. 1994. Systematic studies of Calyceraceae. – Ph.D. diss., Ohio
State University, Columbus, Ohio.
DeVore ML, Stuessy TF. 1995. The place and
time of the origin of the Asteraceae, with additional comments
on the Calyceraceae and Goodeniaceae. – In: Hind DJN,
Jeffrey C, Pope GV (eds), Advances in Compositae systematics, Royal Botanic
Gardens, Kew, pp. 23-40.
DeVore ML, Zhao Z, Jansen RK, Skvarla JJ.
2000. Utility of trends in pollen morphology for phylogenetic analyses: An
example using subfamilies Barnadesioideae and Cichorioideae (Asteraceae). – In: Harley MM,
Morton CM, Blackmore S (eds), Pollen and spores: morphology and biology, Royal
Botanic Gardens, Kew, pp. 399-412.
DeVore ML, Zhao Z, Jansen RK, Skvarla JJ.
2007. Pollen morphology and ultrastructure of Calyceraceae. – Lundellia 10:
32-48.
Devos N, Barker NP, Nordenstam B, Mucina L.
2010. A multi-locus phylogeny of Euryops (Asteraceae, Senecioneae) augments
support for the “Cape to Cairo” hypothesis of floral migrations in Africa.
– Taxon 59: 57-67.
De Waal HL. 1940. Senecio alkaloids III.
Chemical investigations upon the Senecio species responsible for
“bread poisoning”. The isolation of senecionine from Senecio
ilicifolius Thunb. and a new alkaloid, rosmarinine, from Senecio
rosmarinifolius Linn. – Onderstepoort J. Vet. Sci. Anim. Ind. 15:
241-249.
Dias de Morães M, Semir J. 2009. A revision
of Brazilian Dimerostemma (Asteraceae, Heliantheae, Ecliptinae),
with a new species and taxonomic adjustments. – Brittonia 61: 341-365.
Diaz de la Guardia C, Blanca G. 1988a. El
género Geropogon L. (Compositae, Lactuceae). – Lazaroa 9: 31-44.
Diaz de la Guardia C, Blanca G. 1988b. La
posición sistemática de Geropogon L. en la subtribu Scorzonerinae
Dumort. – Lagascalia 15 (Extra): 361-367.
Diaz de la Guardia C, Blanca G. 2004. A new
Spanish species of Tragopogon (Asteraceae: Lactuceae). – Bot. J.
Linn. Soc. 146: 505-511.
Diazgranados M. 2012. A nomenclator for the
frailejones (Espeletiinae Cuatrec., Asteraceae). – PhytoKeys 16:
1-52.
Dickison WC. 1986. Wood anatomy and
affinities of the Alseuosmiaceae. – Syst. Bot.
11: 214-221.
Dickison WC. 1989. Stem and leaf anatomy of
the Alseuosmiaceae. – Aliso
12: 567-578.
Diels L. 1898. Campanulaceae africanae. – Engl.
Bot. Jahrb. Syst. 26: 111-119.
Diels L, Pritzel E. 1905. Stylidiaceae. – Engl. Bot. Jahrb.
Syst. 35: 582-599.
DiLaurenzio L, Struwe L, Pepper AS, Kizirian
D, Albert VA. 1997. Gene expression analysis of sepal identity in
Clermontia (Lobelioideae: Campanulaceae): homeosis and
floral diversification in the Hawaiian archipelago. – In: Evolution of Plant
Development, Keystone symposium B1, Taos, New Mexico, p. 24.
Dillenberger MS, Kadereit JW. 2013. The
phylogeny of the European high mountain genus Adenostyles (Asteraceae-Senecioneae) reveals that
edaphic shifts coincide with dispersal events. – Amer. J. Bot. 100:
1171-1183.
Dillon MO. 2000. Classification and phylogeny
of the South American Gnaphalieae (Asteraceae). – In: Andean Botanical
information System. https://urlproxy.sunet.se/canit/urlproxy.php?_q=aHR0cDovL3d3dy5zYWNoYS5vcmcvR25hcGhhbGllYWUvR25hcGhhbGllYWUuaHRt&_s=ZGVmYXVsdA%3D%3D&_c=f2e6501e&_r=c3Utc2U%3D
Dillon MO. 2003. New combinations in
Luciliocline with notes on South American Gnaphalieae. – Arnaldoa 10:
45-60.
Dillon MO, Sagástegui-Alva A. 1985. New
species and combinations in Belloa (Inuleae-Asteraceae). – Phytologia 58:
392-400.
Dillon MO, Sagástegui-Alva A. 1986.
Jalcophila, a new genus of Andean Inuleae (Asteraceae). – Brittonia 38:
162-167.
Dillon MO, Sagástegui-Alva A. 1992. Sinopsis
de los géneros de Gnaphaliinae (Asteraceae-Inuleae) de Sudamérica.
– Arnaldoa 1: 5-91.
Dillon MO, Turner BL. 1982. Chromosome
numbers of Peruvian Compositae. – Rhodora 84: 131-137.
Dillon MO, Zapata Cruz M. 2010.
Angeldiazia weigendii (Asteraceae, Senecioneae), a new genus
and species from northern Peru. – Arnaldoa 17: 19-24.
Dillon MO, Funk VA, Robinson H, Chan R. 2009.
Liabeae. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics,
evolution, and biogeography of Compositae, International Association for Plant
Taxonomists, Wien, pp. 417-437.
Dimitrova D, Greilhuber J. 2000. Karyotype
and DNA-content evolution in ten species of Crepis (Asteraceae) distributed in Bulgaria.
– Bot. J. Linn. Soc. 132: 281-297.
Dimon M. 1971. Problèmes généraux
soulevés par l’étude pollinique de Composées méditerranéennes. –
Natur. Monspeliensia 22: 129-144.
Dittrich M. 1968a. Karpologische
Untersuchungen zur Systematik von Centaurea und verwandten Gattungen.
– Bot. Jahrb. Syst. 88: 70-162.
Dittrich M. 1968b. Fruchtanatomische und
Zytologische Untersuchungen an einigen Arten der Gattung Rhaponticum
Adans. und Leuzea DC. (Compositae). – Österr. bot. Zeitschr. 115:
379-390.
Dittrich M. 1970. Morphologische und
anatomische Untersuchungen an Früchten der Carduinae (Compositae) I.
Morphologischer Teil. – Candollea 25: 45-67.
Dittrich M. 1971. Lamyropsis
(Charadze) Dittrich. Zur Frucht- und Blütenmorphologie einer kritischen Gruppe
aus der Ptilostemon-Verwandschaft. – Candollea 26: 97-102.
Dittrich M. 1977. Cynareae – systematic
review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae, vol. 2, Academic Press, London, pp. 999-1015.
Dittrich M. 1996. Die Bedeutung
morphologischer und anatomischer Achänen-Merkmale für die Systematik der
Tribus Echinopseae Cass. und Carlineae Cass. – Boissiera 51: 5-102.
Djavadi SB, Attar F. 2005. Sect.
Lasiandra from genus Cousinia Cass. (Compositae) with
emphasis to a new species from east of Iran. – Feddes Repert. 116:
285-289.
Djavadi SB, Attar F. 2006. Two new species of
Cousinia Cass. of sect. Stenocephalae Bunge from Iran. –
Feddes Repert. 117: 453-458.
Djavadi SB, Attar F. 2009. Cousinia
kilouyensis (Asteraceae,
Cardueae), a new endemic species from SW Iran. – Willdenowia 39: 89-92.
Djavadi SB, Attar F, Eskandari M. 2009.
Section Neurocentrae Bunge from genus Cousinia Cass. (Asteraceae) in Iran. – Feddes
Repert. 120: 27-34.
Doll R. 1982. Grundriss der Evolution der
Gattung Taraxacum Zinn. – Feddes Repert. 93: 481-624.
Dominguez XA, Roehill de la Fuente E. 1973.
Sakuranetin and pulcherryl acetate from Eupatorium havanense. –
Phytochemistry 12: 2060.
Dop P. 1912. Gentianacées nouvelles de
l’Indo-Chine. – Bull. Soc. Bot. France 59: 145-147.
Dormer KJ. 1962. The fibrous layers in the
anthers of the Compositae. – New Phytol. 61: 150-163.
Dostál J. 1973. Preliminary notes on the
subtribe Centaureinae. – Acta Bot. Acad. Sci. Hung. 19: 73-79.
Dostál J. 1976. New nomenclatural
combinations and taxa of the Compositae subtribe Centaureinae in Europe. –
Bot. J. Linn. Soc. 71: 191-210.
Downum KR, Keil DJ, Rodriguez E. 1985.
Distribution of acetylenic thiophenes in the Pectidinae. – Biochem. Syst.
Ecol. 13: 109-113.
Dowrick GJ. 1952. Chromosomes of
Chrysanthemum I. The species. – Heredity 6: 365-375.
Dravitzky PV. 1967. A comparative study of
the wood anatomy and floral vascular systems of the New Zealand genera of the
Escalloniaceae,
Carpodetus, Ixerba and Quintinia. – M.Sc. thesis,
University of Canterbury, Christchurch, New Zealand.
Drury DG. 1970. A fresh approach to the
classification of the genus Gnaphalium with particular reference to
the species present in New Zealand (Inuleae-Compositae). – New Zealand J.
Bot. 8: 222-248.
Drury DG. 1971. The American spicate cudweeds
adventive to New Zealand (Gnaphalium Section
Gamochaeta-Compositae). – New Zealand J. Bot. 9: 157-185.
Drury DG. 1975. A comparison of Senecio
kirkii (New Zealand) and Senecio insularis (Lord Howe Island)
with senecios endemic to the island of St. Helena. – New Zealand J. Bot. 13:
769-780.
Drury DG, Watson L. 1965. Anatomy and the
taxonomic significance of gross vegetative morphology in Senecio. –
New Phytol. 64.
Drury DG, Watson L. 1966. Taxonomic
implications of a comparative anatomical study of Inuloideae-Compositae. –
Amer. J. Bot. 53: 828-833.
Duigan SL. 1961.Studies of pollen grains of
plants native to Victoria, Australia I. Goodeniaceae (including
Brunonia). – Proc. Roy. Soc. Victoria, N. S., 74: 87-109.
Duistermaat H. 1996. Monograph of
Arctium L. (Asteraceae).
Generic delimitation (including Cousinia Cass. p. p.), revision of the
species, pollen morphology, and hybrids. – Gorteria, Suppl. 3: 1-143.
Duistermaat H. 1997. Arctium getting
entangled to Cousinia. – Bocconea 5: 1-5.
Dulberger R, Ornduff R. 2000. Stigma
morphology in distylous and non-heterostylous species of Villarsia (Menyanthaceae). – Plant Syst.
Evol. 225: 171-184.
Duman H, Anderberg AA. 1999. An undescribed
species of Pentanema Cass. (Asteraceae-Inuleae) from Turkey; with
notes on the phylogenetic status of the genus. – Bot. J. Linn. Soc. 129:
333-338.
Dunbar A. 1973a. A short report on the fine
structure of some Campanulaceae
pollen. – Grana 13: 25-28.
Dunbar A. 1973b. Pollen ontogeny in some
species of Campanulaceae. A
study by electron microscopy. – Bot. Not. 126: 277-315.
Dunbar A. 1975a. On pollen of Campanulaceae and related families
with special reference to the surface ultrastructure I. Campanulaceae subfam.
Campanuloid[e]ae. – Bot. Not. 128: 73-101.
Dunbar A. 1975b. On pollen of Campanulaceae and related families
with special reference to the surface ultrastructure II. Campanulaceae subfam. Cyphioideae
and subfam. Lobelioideae; Goodeniaceae; Sphenocleaceae. – Bot.
Not. 128: 102-118.
Dunbar A. 1978. Pollen morphology and
taxonomic position of the genus Pentaphragma Wall. (Pentaphragmataceae): the use
of compound fixatives. – Grana 17: 141-147.
Dunbar A. 1981. The preservation of soluble
material on the surface and in cavities of the pollen wall of Campanulaceae and Pentaphragmataceae. –
Micron 12: 47-64.
Dunbar A. 1984. Pollen morphology in Campanulaceae IV. – Nord. J.
Bot. 4: 1-19.
Dunbar A, Wallentinus H-G. 1976. On pollen of
Campanulaceae III. A numerical
taxonomic investigation. – Bot. Not. 129: 69-72.
Dunlop CR. 1980. A revision of
Ixiochlamys (Asteraceae-Astereae). – J. Adelaide
Bot. Gard. 2: 241-252.
Dunlop CR. 1981a. A revision of the genus
Streptoglossa (Asteraceae: Inuleae). – J. Adelaide
Bot. Gard. 3: 167-182.
Dunlop CR. 1981b. Allopterigeron, a
new genus in Asteraceae (Inuleae).
– J. Adelaide Bot. Gard. 3: 183-186.
Duran A, Hamzaoglu E. 2005. Psephellus
turcicus sp. nov. (Asteraceae), a new chasmophyte
species from Central Anatolia, Turkey. – Bot. J. Linn. Soc. 148: 495-500.
Dutta S. 1939. A note on the development of
female gametophyte in some Indian Compositae. – Curr. Sci. 8: 471-472.
Eastwood A, Gibby M, Cronk QCB. 2004.
Evolution of St Helena arborescent Astereae (Asteraceae): relationships of the
genera Commidendrum and Melanodendron. – Bot. J. Linn. Soc.
144: 69-83.
Eddie WMM. 1984. A systematic study of the
genus Musschia Dumortier, with reference to character diversity and
evolution in the Campanulaceae:
Campanuloideae. – M.Sc. thesis, University of Reading, England.
Eddie WMM. 1997. A global reassessment of the
generic relationships in the bellflower family (Campanulaceae). – Ph.D. diss.,
University of Edinburgh.
Eddie WMM, Shulkina T, Gaskin JF, Haberle RC,
Jansen RK. 2003. Phylogeny of Campanulaceae s. str. inferred
from ITS sequences of nuclear ribosomal DNA. – Ann. Missouri Bot. Gard. 90:
554-575.
Eddie WMM, Cupido CN, Skvarla JJ. 2010.
Pollen and reproductive morphology of Rhigiophyllum and
Siphocodon (Campanulaceae): two unique genera
of the fynbos vegetation of South Africa. – Bothalia 40: 103-115.
Edwards RD, Cantley JT, Chau MM, Keeley SC,
Funk VA. 2018. Biogeography and relationships within the Melanthera
alliance: a pan-tropical lineage (Compositae: Heliantheae: Ecliptinae). –
Taxon 67: 552-564.
Egeröd K, Ståhl B. 1991. Revision of
Lycoseris (Compositae-Mutisieae). – Nord. J. Bot. 11: 549-574.
Ehrendorfer F. 1964. Notizen zur
Cytotaxonomie und Evolution der Gattung Artemisia. – Österr. Bot.
Zeitschr. 111: 84-142.
Ehrendorfer F, Guo Y-P. 2005. Changes in the
circumscription of the genus Achillea (Compositae-Anthemideae) and its
subdivision. – Willdenowia 35: 49-54.
Ehrendorfer F, Guo Y-P. 2006.
Multidisciplinary studies on Achillea sensu lato
(Compositae-Anthemideae): new data on systematics and phylogeography. –
Willdenowia 36 (Spec. issue): 69-87.
Ehrendorfer F, Schweizer D, Greger H,
Humphries C. 1977. Chromosome banding and synthetic systematics in
Anacyclus (Asteraceae-Anthemideae). – Taxon
26: 387-394.
Eig A. 1942. Revision of the
Onopordon species of Palestine, Syria and adjacent countries. –
Palestine J. Bot. (Jerusalem), Ser. 2(4): 185-199.
Ekenäs C, Baldwin BG, Andreasen K. 2007. A
molecular phylogenetic study of Arnica (Asteraceae): low chloroplast DNA
variation and problematic subgeneric classification. – Syst. Bot. 32:
917-928.
Eldenäs P. 1990. The genus
Perralderia Cosson (Asteraceae-Inuleae-Inulinae). –
Bot. J. Linn. Soc. 102: 157-173.
Eldenäs P, Anderberg AA. 1995. A cladistic
analysis of Anisopappus (Asteraceae: Inuleae). – Plant Syst.
Evol. 199: 167-192.
Eldenäs P, Anderberg AA. 1999. Phylogenetic
placement and circumscription of tribes Inuleae s. str. and Plucheeae (Asteraceae): evidence from sequences
of chloroplast gene ndhF. – Mol. Phylogen. Evol. 13: 50-58.
Eldenäs P, Källersjö M, Anderberg AA.
1998. Molecular phylogenetics of the tribe Inuleae s. str. (Asteraceae), based on ITS sequences
of nuclear ribosomal DNA. – Plant Syst. Evol. 210: 159-173.
Eldenäs P, Källersjö M, Anderberg AA.
1999. Phylogenetic placement and circumscription of tribes Inuleae s. str. and
Plucheeae (Asteraceae): evidence
from sequences of chloroplast gene ndhF. – Mol. Phylogen. Evol. 13:
50-58.
El-Garf IIA, Osman AK. 2007. Palynological
studies on Egyptian species of subfamily Asteroideae-Compositae (tribes
Astereae, Calenduleae and Eupatorieae). – Feddes Repert. 118: 192-205.
El-Ghazaly G. 1980. Palynology of
Hypochoeridinae and Scolyminae (Compositae). – Opera Bot. 58: 1-48.
El-Ghazaly G, Anderberg AA. 1995. Pollen
morphology in Phagnalon and Aliella (Asteraceae, Gnaphalieae) and its
taxonomical implications. – Grana 34: 89-99.
Eliasson U. 1971. Studies in Galápagos
plants X. The genus Lecocarpus Decaisne. – Svensk Bot. Tidskr. 65:
245-277.
Eliasson U. 1974. Studies in Galápagos
plants XIV. The genus Scalesia Arn. – Opera Bot. 36: 1-117.
Elisens WJ, Boyd RD, Wolfe AD. 1992. Genetic
and morphological divergence among varieties of Aphanostephus
skirrhobasis (Asteraceae-Astereae) and related
species with different chromosome numbers. – Syst. Bot. 17: 380-394.
Ellison WL. 1964. A systematic study of the
genus Bahia (Compositae). – Rhodora 66: 67-86, 177-215, 281-311.
Ellison WL. 1971. Taxonomy of
Platyschkuhria (Compositae). – Brittonia 23: 269-279.
Ellison WL, Alston RE, Turner BL. 1962.
Methods of presentation of crude biochemical data for systematic purposes, with
particular reference to the genus Bahia (Compositae). – Amer. J.
Bot. 49: 599-604.
Engler A. 1891. Saxifragaceae.
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W.
Engelmann, Leipzig, pp. 41-93.
Engler A. 1897. Campanulaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien, Nachträge zu IV(5), W.
Engelmann, Leipzig, pp. 319-320.
Engler A. 1930. Saxifragaceae.
– In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl.,
Bd. 18a, W. Engelmann, Leipzig, pp. 74-226.
Englund M, Pompongrungrueng P, Gustafsson
MHG, Anderberg AA. 2009. Phylogenetic relationships and generic delimitation in
Inuleae subtribe Inulinae (Asteraceae) based on ITS and cpDNA
sequence data. – Cladistics 25: 319-352.
Enke N, Gemeinholzer B. 2008. Babcock
revisited: new insights into generic delimitation and character evolution in
Crepis L. (Compositae: Cichorieae) from ITS and matK. –
Taxon 57: 756-768.
Enke N, Kilian N, Nemomissa S, Gemeinholzer
B. 2008. Afro-alpine Dianthoseris actually a congener of
Crepis s. str. (Compositae, Cichorieae, Crepidinae). – Bot. Jahrb.
Syst. 127: 389-405.
Erbar C. 1992. Floral development of two
species of Stylidium (Stylidiaceae) and some remarks on
the systematic position of the family Stylidiaceae. – Can. J. Bot. 70:
258-271.
Erbar C. 1993. Studies on the floral
development and pollen presentation in Acicarpha tribuloides with a
discussion of the systematic position of the family Calyceraceae. – Bot. Jahrb. Syst.
115: 325-350.
Erbar C. 1997. Fieberklee und Seekanne –
Enzian- oder Aster-verwandt? Zur Blütenentwicklung und systematischen Stellung
der Menyanthaceae. – Bot.
Jahrb. Syst. 119: 115-135.
Erbar C. 2015. Bi- to multi-seriate stylar
hairs in Eremothamneae, Oldenburgieae, Stifftieae, and Wunderlichieae (Asteraceae). – Syst. Bot. 40:
1144-1158.
Erbar C. 2016. Unique style morphology in the
monotypic Famatinanthoideae-Famatinantheae, a recently established subfamily
and tribe of Asteraceae. – Syst.
Bot. 41: 796-806.
Erbar C, Leins P. 1988. Studien zur
Blütenentwicklung und sekundären Pollenpräsentation bei Brunonia
australis Smith (Brunoniaceae). – Bot. Jahrb. Syst. 110: 263-282.
Erbar C, Leins P. 1989. On the early floral
development and the mechanism of secondary pollen presentation in
Campanula, Jasione, and Lobelia. – Bot. Jahrb.
Syst. 111: 29-55.
Erbar C, Leins P. 1995. Portioned pollen
release and syndromes of secondary pollen presentation in the Campanulales-Asterales complex. –
Flora 190: 323-338.
Erbar C, Leins P. 2000. Some interesting
features in the capitulum and flower of Arnoldoa macbrideana Ferreyra
(Asteraceae, Barnadesioideae). –
Bot. Jahrb. Syst. 122: 503-515.
Erbar C, Leins P. 2015a. Styles and new
stigma characters in Mutisieae s. str. (Asteraceae-Mutisioideae) in
comparison with genera of traditionally circumscribed Mutisieae. – Plant
Divers. Evol. 131: 363-393.
Erbar C, Leins P. 2015b. Cuticular patterns
on stylar haris in Asteraceae: a
new micromorphological feature. – Intern. J. Plant Sci. 176: 269-284.
Erbar C, Leins P. 2015c. Diversity of styles
and mechanisms of secondary pollen presentation in basal Asteraceae – new insights in
phylogeny and function. – Flora 217: 109-130.
Erbar C, Leins P. 2016. Styles and new stigma
characters in Mutisieae s.str. (Asteraceae-Mutisioideae) in
comparison with genera of traditionally circumscribed Mutisieae. – Plant Div.
Evol. 131(4): doi: 10.1127/pde/2016/0131-0086.
Erdtman G, Metcalfe CR. 1963. Affinities of
certain genera incertae sedis suggested by pollen morphology and vegetative
anatomy III. The Campanulaceous affinity of Berenice argentea Tulasne.
– Kew Bull. 17: 253-256.
Eren Ö. 2007. The genus
Rhaponticoides Vaill. (Asteraceae) in Turkey: a new species
and first key. – Plant Syst. Evol. 267: 13-23.
Erickson R. 1950. Notes on a common trigger
plant with uncommon habits. – West. Aust. Natur. 2: 97-100.
Erickson R. 1958. Triggerplants. – Paterson
Brokensha Pty., Perth.
Erickson R. 1981. Triggerplants. –
University of Western Australia Press, Nedlands.
Erickson R, Willis JH. 1956a. New species and
varieties of Stylidium from Western Australia. – Muelleria 1:
7-20.
Erickson R, Willis JH. 1956b. Critical notes
on Australian Stylidiaceae, with
descriptions of three new species and two new varieties. – Victorian Natur.
72: 130-136.
Erickson R, Willis JH. 1956c. A new
trigger-plant from the Northern Territory. – Victorian Natur. 73: 5-6.
Erickson R, Willis JH. 1966. Some additions
to Australian Stylidiaceae. –
Victorian Natur. 83: 107-112.
Eriksson T. 1990. Reinstatement of the genus
Leucoblepharis Arnott (Asteraceae-Heliantheae). – Bot.
Jahrb. Syst. 112: 167-191.
Eriksson T. 1991. The systematic position of
the Blepharispermum group (Asteraceae-Heliantheae). – Taxon
40: 33-39.
Eriksson T. 1992. The genus
Blepharispermum (Asteraceae, Heliantheae). – Plant
Syst. Evol. 182: 149-227.
Eriksson T. 1993. The genus
Athroisma (Asteraceae,
Heliantheae). – Bot. J. Linn. Soc. 119: 101-184.
Erkara İ, Köse Y, Osoydan K, Yücel E.
2012. Pollen morphology of some endemic Turkish Centaurea L.
(Asgeraceae, section Phaloletis) and their taxonomic value. – Plant
Syst. Evol. 298: 1111-1117.
Everett J, Doust ANL. 1992. Four new
Australian species of Craspedia sens. strict. (Asteraceae: Gnaphalieae). – Telopea
5: 35-38.
Everett J, Thompson J. 1992. New alpine and
subalpine species in Craspedia sens. strict. (Asteraceae: Gnaphalieae). – Telopea
5: 45-51.
Eyde RH. 1966. Systematic anatomy of the
flower and fruit of Corokia. – Amer. J. Bot. 53: 833-847.
Ezcurra C. 1985. Revisión del género
Chuquiraga (Compositae-Mutisieae). – Darwiniana 26: 219-284.
Ezcurra C. 2002. Phylogeny, morphology, and
biogeography of Chuquiraga, an Andean-Patagonian genus of Asteraceae-Barnadesioideae. – Bot.
Rev. 68: 153-170.
Ezcurra C, Ruggiero A, Crisci JV. 1997.
Phylogeny of Chuquiraga Sect. Acanthophyllae (Asteraceae-Barnadesioideae), and the
evolution of its leaf morphology in relation to climate. – Syst. Bot. 22:
151-163.
Fairbrothers DE. 1966. Serological
correspondence of the genus Corokia with taxa of the Cornaceae, Nyssaceae and Garryaceae. –
Amer. J. Bot. 53: 637-638.
Fang N, Yu S, Mabry TJ. 1986. Flavonoids from
Ageratina calophylla. – Phytochemistry 25: 2684-2686.
Fang SD, Rodriguez E. 1984. Sesquiterpene
lactones from Dicoria canescens. – Phytochemistry 23: 2553-2555.
Fayed A-A. 1979. Revision der Grangeinae (Asteraceae-Astereae). – Mitt. Bot.
Staatssamml. München 15: 425-576.
Fayed A-A. 1991. Systematic revision of
Compositae in Egypt 7. Tribe Inuleae: Phagnalon and Leysera.
– Willdenowia 20: 97-102.
Fayed A-A, Mohamed M-E. 1991a. Systematic
revision of Compositae in Egypt 5. Tribe Inuleae: Pulicaria and
related genera. – Willdenowia 20: 81-89.
Fayed A-A, Mohamed M-E. 1991b. Systemtic
revision of Compositae in Egypt 6. Tribe Inuleae: Inula and related
genera. – Willdenowia 20: 91-96.
Fayed A-A, Zareh M. 1988. Systematic revision
of Compositae in Egypt 3. Tribe Inuleae: Filago and Ifloga.
– Willdenowia 17: 115-123.
Fayed A-A, Zareh M. 1989. Systematic revision
of Compositae in Egypt 4. Tribe Inuleae: Gnaphalium and allied genera.
– Willdenowia 18: 445-453.
Feer H. 1890. Beiträge zur Systematik und
Morphologie der Campanulaceen. – Engl. Bot. Jahrb. Syst. 12: 608-621.
Fehlberg SD, Ranker TA. 2007. Phylogeny and
biogeography of Encelia (Asteraceae) in the Sonoran and
Peninsular Deserts based on multiple DNA sequences. – Syst. Bot. 32:
692-699.
Fehrer J, Gemeinholzer B, Chrtek J Jr,
Bräutigam S. 2007. Incongruent plastid and nuclear DNA phylogenies reveal
ancient intergeneric hybridization in Pilosella hawkweeds
(Hieracium, Cichorieae, Asteraceae). – Mol.
Phylogenet.Evol. 42: 347-361.
Fehrer J, Krahulcová A, Krahulec F, Chrtek J
Jr, Rosenbaumová R, Bräutigam S. 2007. Evolutionary aspects in
Hieracium subgenus Pilosella. – In: Hörandl E,
Grossniklaus U, Dijk PJ van, Sharbel TF (eds), Apomixis, evolution, mechanisms
and perspectives, Regnum Veg. 147, pp. 359-390.
Felippe GM, Salgado-Labouriau ML. 1964.
Pollen grains of plants of the ‘Cerrado’ VI. Compositae. Tribus
Heliantheae. – An. Acad. Bras. Ci. 36: 85-101.
Feráková V. 1977. The genus
Lactuca L. in Europe. – Bratislava.
Fernandes A, Queirós M. 1971. Contribution
à la connaissance cytotaxinomique des Spermatophyta du Portugal II.
Compositae. – Bol. Soc. Brot. 45: 5-121.
Fernandes A, Queirós M. 1972. Systèmes
génétiques chez Hedypnois Scop I. Microsporogénèse. – Bol. Soc.
Brot., ser. II, 46: 5-62.
Fernandez IA, Aguilar JF, Panero JL, Feliner
GN. 2001. A phylogenetic analysis of Doronicum (Asteraceae, Senecioneae) based on
morphological, nuclear ribosomal (ITS), and chloroplast (trnL-F)
evidence. – Mol. Phylogen. Evol. 20: 41-64.
Fernández Casas J, Susanna A. 1986.
Monografía de la sección Chamaecyanus Willk. del género
Centaurea L. – Treb. Inst. Bot. Barcelona 10: 5-174.
Ferreira MZ, Zahradníček J, Kadlecová J,
de Sequeira MM, Chrtek Jr J, Fehrer J. 2015. Tracing the evolutionary history
of the little-known Mediterranean-Macaronesian genus Andryala (Asteraceae) by multigene sequencing.
– Taxon 64: 535-551.
Ferreyra R. 1944. Revision del género
Onoseris. – J. Arnold Arbor. 25: 349-395.
Feuer S, Tomb AS. 1977. Pollen morphology and
detailed structure of family Compositae, tribe Cichorieae II. Subtribe
Microseridinae. – Amer. J. Bot. 64: 230-245.
Field BL, Houben A, Timmis JN, Leach CR.
2006. Internal transcribed spacer sequence analyses indicate cytoevolutionary
patterns within Brachycome Cass. (Asteraceae). – Plant Syst. Evol.
259: 39-51.
Fikenscher LH, Hegnauer R. 1977. Über die
cyanogenen Verbindungen bei einigen Compositae, bei den Oliniaceae und in der
Rutaceae-Gattung
Zieria. – Pharmaceut. Weekbl. 112: 11-20.
Finch RA. 1967. Natural chromosome variation
in Leontodon. – Hereditas 22: 359-386.
Findlay GP. 1982. Generation of torque by the
column of Stylidium. – Aust. J. Plant Physiol. 9: 271-286.
Findlay GP, Findlay N. 1975. Anatomy and
movement of the column in Stylidium. – Aust. J. Plant Physiol. 2:
597-621.
Findlay GP, Findlay N. 1989. The structure of
the column in Stylidium. – Aust. J. Bot. 37: 81-101.
Fioretto A, Alfani A. 1988. Anatomy of
succulence and CAM in 15 species of Senecio. – Bot. Gaz. 149:
142-152.
Fischer H, Jensen U. 1990. Phytoserological
investigation of the tribe Cardueae s.l. (Compositae). – Plant Syst. Evol.
[Suppl.] 4: 99-111.
Fischer H, Jensen U. 1992. Utilization of
proteins to estimate relationships in plants: serology; a discussion based on
the Asteraceae-Cichorioideae. –
Belg. J. Bot. 125: 243-255.
Fischer NH, Wiley RA, Lin H, Karimian K,
Politz SM. 1975. New melampodide sesquiterpene lactones from Melampodium
leucanthum. – Phytochemistry 14: 2243-2245.
Fiz O, Valcárcel V, Vargas P. 2002.
Phylogenetic position of Mediterranean Astereae and character evolution of
daisies (Bellis, Asteraceae) inferred from nrDNA ITS
sequences. – Mol. Phylogen. Evol. 25: 157-171.
Fjellheim S, Jørgensen MH, Kjos M, Borgen L.
2009. A molecular study of hybridization and homoploid hybrid speciation in
Argyranthemum (Asteraceae) on Tenerife, the Canary
Islands. – Bot. J. Linn. Soc. 159: 19-31.
Flagel LE, Rapp RA, Grover CE, Widrlechner
MP, Hawkins J, Grafenberg JL, Alvarez I, Chung GY, Wendel JF. 2008.
Phylogenetic, morphological, and chemotaxonomic incongruence in the North
American endemic genus Echinacea. – Amer. J. Bot. 95: 756-765.
Flann CM. 2005. Systematics of
Euchiton (Gnaphalieae: Asteraceae) with a focus on alpine
taxa in Australia and New Zealand. – Ph.D. diss., University of Melbourne,
Victoria, Australia.
Flann CM, Breitwieser I, Ward JM, Walsh NG,
Ladiges PY. 2008. Morphometric study of Euchiton traversii complex
(Gnaphalieae: Asteraceae). –
Aust. Syst. Bot. 21: 178-191.
Font M, Garnatje T, Garcia-Jacas N, Susanna
A. 2002. Delineation and phylogeny of Centaurea sect.
Acrocentron based on DNA sequences: a restoration of the genus
Crocodylium and indirect evidence of introgression. – Plant Syst.
Evol. 234: 15-26.
Ford KA, Ward JM, Smissen RD, Wagstaff SJ,
Breitwieser I. 2007. Phylogeny and biogeography of Craspedia (Asteraceae: Gnaphalieae) based on
ITS, ETS and psbA-trnH sequence data. – Taxon 56:
783-794.
Forster PI. 1990. Argophyllum verae
(Saxifragaceae), a
new species form northern Queensland. – Austrobaileya 3: 173-176.
Förther H, Podlech D. 2002. Die Gattung
Hypochaeris L. sect. Hypochaeris (Compositae) im westlichen
Nordafrika. – Sendtnera 8: 35-43.
Foster RI. 1972. Explosive chromosome
evolution in Downingia yina. – Ph.D. diss., University of
California, Davis, California.
Fraga P, Castro M, Rossello JA. 2007. A new
annual species of Bellium (Asteraceae) from the Balearic
Islands. – Bot. J. Linn. Soc. 154: 65-77.
Frajman B, Schneeweiss GM. 2009. A
campanulaceous fate: the Albanian stenoendemic Asyneuma comosiforme in
fact belongs to isophyllous Campanula. – Syst. Bot. 34: 595-601.
Franchet A. 1888. Les Mutisiacées du
Yun-Nan. – J. Bot. (Morot) 2: 65-71.
Franchet A. 1893. Un Gerbera de la
Chine occidentale. – J. Bot. (Morot) 7: 152-155.
Francisco-Ortega J, Crawford DJ,
Santos-Guerra A, Sa-Fontinha S. 1995. Genetic divergence among Mediterranean
and Macaronesian genera of the subtribe Chrysantheminae (Asteraceae). – Amer. J. Bot. 82:
1321-1328.
Francisco-Ortega J, Jansen RK. Crawford DJ,
Santos-Guerra A. 1995. Chloroplast DNA evidence for intergeneric relationships
of the Macaronesian endemic genus Argyranthemum (Asteraceae). – Syst. Bot. 20:
413-422.
Francisco-Ortega J, Crawford DJ,
Santos-Guerra A, Carvalho JA. 1996. Isozyme differentiation in the endemic
genus Argyranthemum (Asteraceae: Anthemideae) in the
Macaronesian Islands. – Plant Syst. Evol. 202: 137-152.
Francisco-Ortega J, Santos-Guerra A, Hines A,
Jansen RK. 1997. Molecular evidence for a Mediterranean origin of the
Macaronesian endemic genus Argyranthemum (Asteraceae). – Amer. J. Bot. 84:
1595-1613.
Francisco-Ortega J, Crawford DJ,
Santos-Guerra A, Jansen RK. 1997. Origin and evolution of
Argyranthemum (Asteraceae: Anthemideae). – In:
Givnish TJ, Sytsma KJ (eds), Molecular evolution and adaptive radiation,
Cambridge University Press, New York, pp. 407-432.
Francisco-Ortega J, Goertzen LR,
Santos-Guerra A, Benabid A, Jansen RK. 1999. Molecular systematics of the
Asteriscus alliance (Asteraceae: Inuleae) I: evidence from
the internal transcribed spacers of nuclear ribosomal DNA. – Syst. Bot. 24:
249-266.
Francisco-Ortega J, Park S-J, Santos-Guerra
A, Benabid A, Jansen RK. 2001. Origin and evolution of the endemic Macaronesian
Inuleae (Asteraceae): evidence
from the internal transcribed spacers of nuclear ribosomal DNA. – Biol. J.
Linn. Soc. 72: 77-97.
Francisco-Ortega J, Barber JC, Santos-Guerra
A, Febles-Hernandez R, Jansen RK. 2001. Origin and evolution of the endemic
genera of Gonosperminae (Asteraceae: Anathemideae) from the
Canary Islands: evidence from nucleotide sequences of the internal transcribed
spacers of the nuclear ribosomal DNA. – Amer. J. Bot. 88: 161-169.
Franzén R. 1986. Anthemis cretica
(Asteraceae) and related species
in Greece. – Willdenowia 16: 35-45.
Freire SE. 1986a. Novenia: Nuevo
género de Inuleae (Compositae). – Bol. Soc. Argent. Bot. 24: 295-304.
Freire SE. 1986b. Revisión del género
Lucilia (Compositae, Inuleae). – Darwiniana 27: 431-490.
Freire SE. 1986c. Números cromosómicos en
el género Lucilia (Compositae, Inuleae). – Bol. Soc. Argent. Bot.
24: 411-413.
Freire SE. 1987. Nuevos sinónimos y una
nueva combinación en el género Lucilia (Compositae, Inuleae). –
Darwiniana 28: 409-411.
Freire SE. 1989. Oligandra Less. is
Lucilia Cass. (Compositae, Inuleae). – Taxon 38: 298-299.
Freire SE. 1993. A revision of
Chionolaena (Compositae, Gnaphalieae). – Ann. Missouri Bot. Gard.
80: 397-438.
Freire SE. 2007. Systematic revision and
phylogeny of Ainsliaea DC. (Asteraceae, Mutisieae). – Ann.
Missouri Bot. Gard. 94: 79-191.
Freire SE. 2009. Pertyeae (Pertyoideae). –
In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and
biogeography of Compositae, International Association for Plant Taxonomists,
Wien, pp. 315-326.
Freire SE, Iharlegui L. 1997. Sinopsis
preliminar del género Gamochaeta (Asteraceae, Gnaphalieae). – Bol.
Soc. Argent. Bot. 33: 23-35.
Freire SE, Katinas L. 1995a. Filogenia, forma
y función, un ejemplo en la familia de las Compuestas. – Innovación y
Ciencia 4: 52-57.
Freire SE, Katinas L. 1995b. Morphology and
ontogeny of the cypsela hairs of Nassauviinae (Asteraceae, Mutisieae). – In: Hind
DJN, Jeffrey C, Pope GV (eds), Advances in Compositae Systematics, Royal
Botanic Gardens, Kew, pp. 107-143.
Freire SE, Crisci JV, Katinas L. 1993. A
cladistic analysis of Nassauvia Comm. ex Juss. (Asteraceae, Mutisieae) and related
genera. – Bot. J. Linn. Soc. 112: 293-309.
Freire SE, Katinas L, Sancho G. 2002.
Gochnatia (Asteraceae,
Mutisieae) and the Gochnatia complex: taxonomic implications from
morphology. – Ann. Missouri Bot.Gard. 89: 524-550.
Freire SE, Barboza GE, Cantero JJ, Espinar
LA. 2014. Famatinanthus, a new Andean genus segregated from
Aphyllocladus (Asteraceae). – Syst. Bot. 39:
349-360.
Freire SE, Chemisquy MA, Anderberg AA, Beck
SG, Meneses RI, Loeuille B, Urtubey E. 2015. The Lucilia group (Asteraceae, Gnaphalieae):
phylogenetic and taxonomic considerations based on molecular and morphological
evidence. – Plant Syst. Evol. 301: 1227-1248.
Freitas FS, De-Paula OC, Nakajima JN,
Marzinek J. 2015. Fruits of Heterocoma (Vernonieae-Lychnophorinae):
taxonomic significance and a new pattern of phytomelanin deposition in Asteraceae. – Bot. J. Linn. Soc.
179: 255-265.
Fries RE. 1925a. Die Sonchus-Arten
des tropischen und südlichen Afrika. – Acta Horti Berg. 8: 89-121.
Fries RE. 1925b. Die Gattung Tolpis
im tropischen Afrika. – Acta Horti Berg. 8: 269-273.
Fries RE, Fries TCE. 1922a. Über die
Riesen-Senecionen der afrikanischen Hochgebirge. – Svensk Bot. Tidskr. 16:
321-340.
Fries RE, Fries TCE. 1922b. Die
Riesen-Lobelien Afrikas. – Svensk Bot. Tidskr. 16: 383-416.
Fritsch K. 1891a. Caprifoliaceae. – In:
Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(4), W.
Engelmann, Leipzig, pp. 156-169.
Fritsch R. 1935. Zytologische Untersuchungen
der Gattung Centaurea. – Jena.
Fu Z-X, Jiao B-H, Nie B, Zhang G-J, Gao T-G,
China Phylogeny Consortium. 2016. A comprehensive generic-level phylogeny of
the sunflower family: implications for the systematics of Chinese Asteraceae. – J. Syst. Evol. 54:
416-437.
Fujikawa K, Ohba H. 2003. A list of
chromosome numbers of Saussurea (Asteraceae). – Newslett. Himalayan
Bot. 32: 9-14.
Fujikawa K, Ikeda H, Murata K, Kobayashi T,
Nakano T, Ohba H, Wu SG. 2004. Chromosome numbers of fifteen species of the
genus Saussurea DC. (Asteraceae) in the Himalayas and the
adjacent region. – J. Jap. Bot. 79: 271-280.
Funk VA. 1982. The systematics of
Montanoa (Asteraceae,
Heliantheae). – Mem. New York Bot. Gard. 36: 1-133.
Funk VA. 1985. Cladistics and generic
concepts in the Compositae. – Taxon 34: 72-80.
Funk VA. 2009. A new tribe Platycarpheae and
a new genus Platycarphella in the Cichorioideae (Compositae or Asteraceae). – Compositae
Newsletter 47: 24-27.
Funk VA, Chan RA. 2003. A brief survey of the
phylogeny of the Arctoteae (Compositae: Cichorioideae s.s.). – Comp.
Newslett. 40: 13-14.
Funk VA, Chan R. 2008. Phylogeny of the spiny
African daisies (Compositae, tribe Arctotideae, subtribe Gorteriinae) based on
trnL-F, ndhF, and ITS sequence data. – Mol. Phylogen. Evol.
48: 47-60.
Funk VA, Chan R. 2009. Introduction to
Cichorioideae. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 335-342.
Funk VA, Fragman-Sapir O. 2009. Gymnarrheneae
(Gymnarrhenoideae). – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 327-332.
Funk VA, Hind DJN. 2009. Hecastocleideae
(Hecastocleidoideae). – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 261-265.
Funk VA, Jeffrey C. 1984. The systematics of
Montanoa (Asteraceae,
Heliantheae). – Kew Bull. 38: 677-680.
Funk VA, Karis PO. 2009.
Heterolepis: an unplaced genus. – In: Funk VA, Susanna A, Stuessy
TF, Bayer RJ (eds), Systematics, evolution, and biogeography of Compositae,
International Association for Plant Taxonomists, Wien, pp. 483-486.
Funk VA, Koekemoer M. 2011. A monograph of
the small tribe Platycarpheae (Compositae: Cichorioideae). – Syst. Bot. 36:
191-208.
Funk VA, Robinson H. 2001. A bully new genus
from the Andes (Compositae: Liabeae). – Syst Bot. 26: 216-225.
Funk VA, Robinson H. 2009. Sampera,
a new genus of Liabeae (Compositae or Asteraceae) from the northern Andes.
– Proc. Biol. Soc. Washington 122: 155-161.
Funk VA, Robinson H. 2017. Nine species from
Madagascar are moved from Vernonia to Distephanus
(Compositae, Vernonieae). – PhytoKeys 77: 89-92.
Funk VA, Roque N. 2011. The monotypic Andean
genus Fucaldea (Compositae, Barnadesioideae) gains a new species from
northeastern Brazil. – Taxon 60: 1095-1103.
Funk VA, Zermoglio MF. 1999. A revision of
Chrysactinium (Compositae: Liabeae). – Syst. Bot. 24: 323-338.
Funk VA, Robinson H, McKee GS, Pruski JF.
1995. Neotropical montane Compositae with an emphasis on the Andes. – In:
Churchill SP, Balslev H, Forero E, Luteyn JL (eds), Biodiversity and
conservation of Neotropical montane forests, New York Botanical Garden, New
York, pp. 451-471.
Funk VA, Robinson H, Dillon MO. 1996.
Liabeae: taxonomy, phylogeny and biogeography. – In: Hind DJN, Beentje HJ
(eds), Compositae: systematics, Proceedings of the International Compositae
Conference, Kew (1994), Royal Botanic Gardens, Kew, 1: 545-567.
Funk VA, Chan R, Keeley S. 2004. Insights
into the evolution of the tribe Arctot[id]eae (Compositae: subfamily
Cichorioideae s.s.) using trnL-F, ndhF, and ITS. – Taxon
53: 637-655.
Funk VA, Bayer RJ, Keeley S, Chan R, Watson
L, Gemeinholzer B, Schilling EE, Panero JL, Baldwin BG, Garcia-Jacas NT,
Susanna A, Jansen RK. 2005. Everywhere but Antarctica: using a supertree to
understand the diversity and distribution of the Compositae. – In: Friis I,
Balslev H (eds), Plant diversity and complexity patterns – local, regional
and global dimensions. Proceedings of an international symposium, Royal Danish
Academy of Sciences and Letters, Copenhagen, 25-28 May 2003, Biol. Skr. 55:
343-374.
Funk VA, Chan R, Holland A. 2007.
Cymbonotus (Compositae: Arctotideae, Arctotidinae): an endemic
Australian genus embedded in a southern African clade. – Bot. J. Linn. Soc.
153: 1-8.
Funk VA, Susanna A, Stuessy TF, Bayer RJ
(eds). 2009. Systematics, evolution, and biogeography of Compositae. –
International Association for Plant Taxonomists, Wien.
Funk VA, Susanna A, Stuessy TF, Robinson H.
2009. Classification of Compositae. – In: Funk VA, Susanna A, Stuessy TF,
Bayer RJ (eds), Systematics, evolution, and biogeography of Compositae,
International Association for Plant Taxonomists, Wien, pp. 171-189.
Funk VA, Koekemoer M, Robinson H, Skvarla JJ.
2009. Platycarpheae. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 471-476.
Funk VA, et al. 2009. Compositae metatrees:
the next generation. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 747-777.
Funk VA, Kelloff C, Chan R. 2012. Phylogeny
and biogeography of the tribe Liabeae (Compositae; subfamily Cichorioideae).
– Taxon 61: 437-455.
Funk VA, Sancho G, Roque N, Kelloff CL,
Ventosa-Rodríguez I, Diazgranados M, Bonifacino JM, Chan R. 2014. A phylogeny
of the Gochnatieae: understanding a critically placed tribe in the Compositae.
– Taxon 63: 859-882.
Funk VA, Pasini E, Bonifacino JM, Katinas L.
2016. Home at last: the enigmatic genera Eriachaenium and
Adenocaulon (Compositae, Mutisioideae, Mutisieae, Adenocaulinae). –
PhytoKeys 60: 1-19.
Funk VA, Sancho G, Roque N. 2017.
Nahuatlea: a new genus of Compositae (Gochnatieae) from North America.
– PhytoKeys 91: 105-124.
Funston AM. 2008. Taxonomic revision of
Roldana (Asteraceae:
Senecioneae), a genus of the southwestern U.S.A., Mexico, and Central America.
– Ann. Missouri Bot. Gard. 95: 282-337.
Furuya T, Murakami K, Hikichi M. 1971.
Senkirkine, a pyrrolizidine alkaloid from Farfugium japonicum. –
Phytochemistry 10: 3306-3307.
Gabrieljan E. 1988. Synopsis specierum
generis Centaurea L. (Asteraceae) subgeneris
Xanthopsis (DC.) Tzvelev. – Novosti Sist. Vysš. Rast. 25: 160-171.
[In Russian]
Gabrieljan E. 1994. New taxa of the subgenus
Psephellus of the genus Centaurea from Armenia and Iran. –
Bot. Žurn. 79: 120-129. [In Russian]
Gabrieljan E, Handžjan N. 1986. Obzor
polynei (Artemisia L., Asteraceae) Juznogo Zakavkazja. –
Nov. Sit. Vysš. Rast. 23: 206-217.
Gabrieljan E, Jarvis CE. 1996. Amberboa
moschata, A. glauca, and A. amberboi (Asteraceae: Cardueae). A note on
their taxonomy and typification of their names. – Taxon 45: 213-215.
Gadek PA, Bruhl JJ, Quinn CJ. 1989. Exine
structure in the ‘Cotuleae’ (Anthemideae, Asteraceae). – Grana 28:
163-178.
Gadella TWJ. 1964. Cytotaxonomic studies in
the genus Campanula. – Wendtia 11: 1-104.
Gadella TWJ. 1966a. Some notes on the
delimitation of genera in the Campanulaceae I. – Proc. Kon.
Nederl. Akad. Wetensch., ser. C, 69: 502-508.
Gadella TWJ. 1966b. Some notes on the
delimitation of genera in the Campanulaceae II. – Proc. Kon.
Nederl. Akad. Wetensch., ser. C, 69: 509-521.
Gadella TWJ. 1972. Biosystematic studies in
Hieracium pilosella L. and some related species of the subgenus
Pilosella. – Bot. Not. 125: 361-369.
Gage D. 1985. Chemical data and their bearing
upon generic delineations in the Eupatorieae. – Taxon 34: 61-71.
Gailing O, Bachmann K. 2002. QTL analysis
reveals different and independent modes of inheritance for diagnostic achene
characters in Microseris (Asteraceae). – Organisms Divers.
Evol. 2: 277-288.
Gaiser LO. 1954. Studies in the Kuhniinae
(Eupatorieae) II. – J. Arnold Arbor. 35: 87-133.
Galán de Mera A, Castro E de, Vicente
Orellana JA. 1999. Hypochaeris alliatae group (Asteraceae) in the western
Mediterranean Region. – Nord. J. Bot. 19: 587-595.
Galbany-Casals M, Romo AM. 2008. Polyploidy
and new chromosome counts in Helichrysum (Asteraceae, Gnaphalieae). – Bot. J.
Linn. Soc. 158: 511-521.
Galbany-Casals M, Garcia-Jacas N, Susanna A,
Sáez L, Benedí C. 2004. Phylogenetic relationships in the Mediterranean
Helichrysum (Asteraceae,
Gnaphalieae) based on nuclear rDNA ITS sequence data. – Aust. Syst. Bot. 17:
241-253.
Galbany-Casals M, Sáez L, Benedí C. 2004.
Taxonomy of Castroviejoa, a new genus of Gnaphalieae (Asteraceae), endemic to the
Mediterranean islands Corsica and Sardinia. – Aust. Syst. Bot. 17:
581-591.
Galbany-Casals M, Garcia-Jacas N, Sáez L,
Benedí C, Susanna A. 2009. Phylogeny, biogeography, and character evolution in
Mediterranean, Asiatic and Macaronesian Helichrysum (Asteraceae, Gnaphalieae) infered from
nuclear phylogenetic analyses. – Intern. J. Plant Sci. 170: 365-380.
Galbany-Casals M, Andrés-Sánchez S,
Garcia-Jacas N, Susanna A, Rico E, Martínez-Ortega MM. 2010. How many of
Cassini anagrams should there be? Molecular systematics and phylogenetic
relationships in the Filago group (Asteraceae, Gnaphalieae), with
special focus on the genus Filago. – Taxon 59: 1671-1689.
Galbany-Casals M, Unwin M, Garcia-Jacas N,
Smissen RD, Susanna A, Bayer RJ. 2014. Phylogenetic relationships in
Helichrysum (Compositae: Gnaphalieae) and related genera: incongruence
between nuclear and plastid phylogenies, biogeographic and morphological
patterns, and implications for generic delimitation. – Taxon 63: 608-624.
Galetto L. 1995. Estudios sobre el nectar y
los nectarios en Hyaloseris rubricunda y Barnadesia odorata
(Asteraceae-Mutisieae). –
Darwiniana 33: 127-133.
Gallego MJ. 1980. Estudio caryológico de las
especies españolas del género Reichardia Roth (Compositae). –
Lagascalia 9: 149-158.
Gallego MJ, Talavera S. 1983. Notas sobre las
especies españolas del género Calendula L. – Lagascalia 11:
100-104.
Galuško A, Alieva A. 1976. Nova species
generis Psephellus Cass. (Asteraceae) e Caucasi boreali. –
Novosti Sist. Vysš. Rast. 13: 246-248. [In Russian]
Gamal-Eldin E. 1981. Revision der Gattung
Pulicaria (Compositae-Inuleae) für Afrika, Makaronesien und Arabien.
– Phanerog. Monogr. 14: 1-311.
Gamal-Eldin E. 1984. Studies in the flora of
Arabia VIII: a new Pulicaria from Oman [Including a Check-List of
Pulicaria in the Arabian Peninsula]. – Notes Roy. Bot. Gard. Edinb.
41: 467-471.
Gamal-Eldin E. 1986. Studies in the flora of
Arabia XVII: further new and interesting taxa of Pulicaria from Oman.
– Notes Roy. Bot. Gard. Edinb. 43: 481-488.
Gamerro JC. 1985. Morfología del polen de
Huarpea y su relación con Barnadesia (Mutisieae,
Compositae). – Darwiniana 26: 43-51.
Gamerro JC. 1990. Identidad de
Pseudostifftia con Moquinia (Compositae) y consideracions
sobre la ubicacion tribal del taxon. – Darwiniana 30: 123-136.
Ganders FR. 1989. Adaptive radiation in
Hawaiian Bidens. – In: Giddings LV, Kaneshiro KY, Anderson WW (eds),
Genetics, speciation, and the founder principle, Oxford University Press,
Oxford, New York, pp. 99-112.
Ganders FR, Nagata KM. 1984. The role of
hybridization in th evolution of Bidens in the Hawaiian Islands. – In: Grant
WF (ed), Plant biosystematics, Academic Press, Don Mills Ontario, Canada, pp.
179-194.
Ganders FR, Bohm BA, McCormick SP. 1990.
Flavonoid variation in Hawaiian Bidens. – Syst. Bot. 15: 231-239.
Ganders FR, Berbee M, Pirseyedi M. 2000. ITS
base sequence phylogeny in Bidens (Asteraceae): evidence for the
continental relatives of Hawaiian and Marquesan Bidens. – Syst. Bot.
25: 122-133.
Gandhi KN. 2006a. Proposal to conserve the
name Picradeniopsis (Asteraceae) with a conserved type.
– Taxon 55: 233-234.
Gandhi KN. 2006b. Proposal to conserve the
name Adenophyllum (Asteraceae). – Taxon 55:
533-534.
Gao T-G, Wang W, Bayer RJ, Li D-Z. 2009.
Systematic position of the enigmatic genus Sheareria (Asteraceae) – evidence from
molecular, morphological and cytological data. – Taxon 58: 769-780.
García J, Scholz S, Hernández E. 1994.
Helichrysum alucense (Compositae), nuevo endemismo de la isla de La
Gomera (Islas Canarias). – Bot. Macaronésica 21: 51-58.
Garcia S, Garnatje T, Hidalgo O, McArthur ED,
Siljak-Yakovlev S, Vallès J. 2007. Extensive ribosomal DNA (18S-5.8S-26S and
5S) colocalization in the North American endemic sagebrushes (subgenus
Tridentatae, Artemisia, Asteraceae) revealed by FISH. –
Plant Syst. Evol. 267: 79-92.
Garcia S, McArthur ED, Pellicer J, Sanderson
SC, Vallès J, Garnatje T. 2011. A molecular phylogenetic approach to western
North America endemic Artemisia and allies (Asteraceae): untangling the
sagebrushes. – Amer. J. Bot. 98: 638-653.
García-Jacas N. 1992. Estudi taxonòmic i
biosistemàtic de les espécies ibériques i nord-africanes del génere
Centaurea sect. Acrocentron subsect. Orientales. –
Ph.D. diss., Universidad de Barcelona, Spain.
García-Jacas N, Susanna A. 1992.
Karyological notes on Centaurea sect. Acrocentron (Asteraceae). – Plant Syst. Evol.
179: 1-18.
García-Jacas N, Susanna A, İlarlsan R.
1996. Aneuploidy in the Centaureinae (Compositae): is n = 7 the end of the
series? – Taxon 45: 39-42.
García-Jacas N, Susanna A, Mozaffarian V.
1998. New chromosome counts in the subtribe Centaureinae (Asteraceae, Cardueae) from West Asia
III. – Bot. J. Linn. Soc. 128: 413-422.
García-Jacas N, Susanna A, Mozaffarian R,
Ilarslan R. 2000. The natural delimitation of Centaurea (Asteraceae: Cardueae): ITS sequence
analysis of the Centaurea jacea group. – Plant Syst. Evol. 223:
185-199.
García-Jacas N, Susanna A. Garnatje T,
Vilatersana R. 2001. Generic delimitation and phylogeny of the subtribe
Centaureinae (Asteraceae): a
combined nuclear and chloroplast DNA analysis. – Ann. Bot. 87: 503-515.
García-Jacas N, Garnatje T, Susanna A,
Vilatersana R. 2002. Tribal and subtribal delimitation and phylogeny of the
Cardueae (Asteraceae): a combined
nuclear and chloroplast DNA analysis. – Mol. Phylogen. Evol. 22: 51-64.
García-Jacas N, Galbany-Casals M,
Romashchenko K, Susanna A. 2008. On the conflicting generic delineation in the
Onopordum group (Compositae, Cardueae-Carduinae): a combined nuclear
and plastid molecular approach. – Aust. Syst. Bot. 21: 301-311.
Gardner AG, Sessa EB, Michener P, Johnson E,
Shepherd KA, Howarth DG, Jabaily RS. 2016. Utilizing next-generation sequencing
to resolve the backbone of the Core Goodeniaceae and inform future
taxonomic and floral form studies. – Mol. Phylogen. Evol. 94: 605-617.
Gardner CA, George AS. 1963.
Neogoodenia. – J. Roy. Soc. Western Australia 46: 138.
Gardner RC. 1976. Evolution and adaptive
radiation in Lipochaeta (Compositae) of the Hawaiian Islands. –
Syst. Bot. 1: 383-391.
Gardner RC. 1977. Observations on tetramerous
disc florets in the Compositae. – Rhodora 79: 139-146.
Gardner RC. 1979. Revision of
Lipochaeta (Compositae. Heliantheae) of the Hawaiian Islands. –
Rhodora 81: 291-343.
Gardner RC, La Duke JC. 1978. Phyletic and
cladistic relationships in Lipochaeta (Compositae). – Syst. Bot. 3:
197-207.
Gardner RO. 1976. Studies in the Alseuosmiaceae. – Ph.D. diss,
University of Auckland, New Zealand.
Gardner RO. 1978a. Systematic notes on the Alseuosmiaceae. – Blumea 24:
138-142.
Gardner RO. 1978b. The species of
Alseuosmia (Alseuosmiaceae). – New Zealand
J. Bot. 16: 271-277.
Gardou C. 1969. Caryosystématique des
Centaurées de la Section Acrocentron Cass. (in Hoffmann 1897). –
Bull. Soc. Bot. France 116: 29-38.
Garg SK, Sastry TCS. 1996. Indian Compositae
in foods and flavours – a review. – In: Caligari PDS, Hind DJN (eds),
Compositae: biology and utilization. Proceedings of the International
Compositae Conference, Kew, 1994, vol. 2, Royal Botanic Gardens, Kew, pp.
361-382.
Garg SK, Sharma KC. 2007. Taxonomical
significance of the morphological and scanning electron microscopic surface
patterns of cypselas in some members of the tribe Heliantheae (Asteraceae). – Feddes Repert. 118:
165-191.
Garnatje T, Martin J. 2007. Pollen studies in
the genus Echinops L. and Xeranthemum group (Asteraceae). – Bot. J. Linn. Soc.
154: 549-557.
Garnatje T, Vilatersana R, Susanna A, Vallés
J, Siljak-Yakovlev S. 2004. Contribution to the karyological knowledge of
Echinops (Asteraceae,
Cardueae) and related genera. – Bot. J. Linn. Soc. 145: 337-344.
Garnatje T, Vallés J, Vilatersana R,
Garcia-Jacas N, Susanna A, Siljak-Yakovlev S. 2004. Molecular cytogenetics of
Xeranthemum L. and related genera (Asteraceae, Cardueae). – Plant
Biol. 6: 140-146.
Garnatje T, Garcia S, Canela MÁ. 2007.
Genome size variation from a phylogenetic perspective in the genus
Cheirolophus Cass. (Asteraceae): biogeographic
implications. – Plant Syst. Evol. 264: 117-134.
Gaskin JF, Wilson LM. 2007. Phylogenetic
relationships among native and naturalized Hieracium (Asteraceae) in Canada and the United
States based on plastid DNA sequences. – Syst. Bot. 32: 478-485.
Gatt MK, Hammett KRW, Murray BG. 2000.
Molecular phylogeny of the genus Dahlia Cav. (Asteraceae,
Heliantheae-Coreopsidinae) using sequences derived from the internal
transcribed spacers of nuclear ribosomal DNA. – Bot. J. Linn. Soc. 133:
229-239.
Gattuso M, Gattuso S. 1989. Exomorfologia y
anatomia de Nymphoides indica (L.) O. Kuntze (Menyanthaceae). – Parodiana 5:
249-259.
Gautier L. 1992. Taxonomy and distribution of
a tropical weed: Chromolaena odorata (L.) R. King & H. Robinson.
– Candollea 47: 645-662.
Geleta M, Bryngelsson T, Bekele E, Dagne K.
2007. Comparative analysis of genetic relationship and diagnostic markers of
several taxa of Guizotia Cass. (Asteraceae) as revealed by AFLPs and
RAPDs. – Plant Syst. Evol. 265: 221-239.
Gentscheff G. 1937. Zytologische und
embryologische Studien über einige Hieracium-Arten. – Planta 27:
165-195.
Georgiadis T. 1981. Contribution à
l’étude phylogénétique du genre Centaurea L. (section
Acrolophus (Cass.) DC.) en Grèce. – Thèse, Aix-Marseille-I.
Georgiadis T, Phitos D. 1976. Contribution à
l’étude cytotaxonomique du genre Centaurea L. (Sectio
Acrolophus (Cass.) DC.) en Grèce. – Biol. & Écol. Médit. 3:
13-16.
Geslot A, Médus J. 1974. Quelques remarques
sur les rélations entre morphologie pollinique et polyploïdie dans le genre
Campanula sous-section Heterophylla. – Rev. Palaeobot.
Palynol. 17: 233-243.
Ghaffari SM, Attar F, Ghahreman A. 2000.
Distribution and chromosome studies on some species of Cousinia Cass.
(section Cynaroideae) from Iran. – Pakistan J. Bot. 3: 311-316.
Ghaffari SM, Garcia-Jacas N, Susanna A. 2006.
New chromosome counts in the genus Cousinia (Asteraceae) from Iran. – Bot. J.
Linn. Soc. 151: 411-419.
Ghafoor A, Qaiser M, Abid R. 2017. Notes on
Cichoreae (Asteraceae) from
Pakistan and Kashmir: some additions and corrections. – Pak. J. Bot. 49:
1323-1325.
Ghimire B, Jeong MJ, Lee KM, Heo K, Lee CH,
Suh GU. 2016. Achene morphology of Saussurea species (Asteraceae, Carduee) in Korea and its
systematic implications. – Bot. J. Linn. Soc. 181: 692-710.
Ghosh RB. 1974. A contribution to the
embryology of Eupatorium odoratum together with discussion on its
interrelationships. – Broteria, Ser. Ci. Nat. 43: 103-117.
Gibson N. 2014. An amphibious
Goodenia (Goodeniaceae)
from an ephemeral arid zone wetland. – Nuytsia 24: 23-28.
Gilbert MG. 1981. Notes on East African
Vernonieae (Compositae) 1-3. – Kew Bull. 36: 591-596.
Gilbert MG. 1986. Notes on East African
Vernonieae (Compositae) 4. A revision of the Vernonia galamensis
complex. – Kew Bull. 41: 19-35.
Gilbert MG, Jeffrey C. 1988. A revision of
Ethulia (Compositae: Vernonieae). – Kew Bull. 43: 165-193.
Gilg E. 1895. Gentianaceae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien, IV(2), W. Engelmann,
Leipzig, pp. 50-108.
Gill LS, Omoigui DI. 1992. Chromosome numbers
in some Nigerian Compositae. – Compositae Newslett. 20-21: 12-15.
Gillett GW, Lim EKS. 1970. An experimental
study of the genus Bidens (Asteraceae) in the Hawaiian Islands.
–Univ. Calif. Publ. Bot. 56: 1-63.
Gillett JM. 1968. The systematics of the
Asiatic and American populations of Fauria crista-galli (Menyanthaceae). – Can. J. Bot.
46: 92-96.
Giroux M. 1930. Sur la carpologie de quelques
Composées nord-africaines. – Bull. Soc. Hist. Nat. Afrique N. 21:
161-189.
Giuliano DA. 2001. Clasificación
infragenérica de las especies Argentinas de Baccharis (Asteraceae, Astereae). – Darwiniana
39: 131-154.
Given DR. 1969. Taxonomic notes on the genus
Celmisia (Compositae). A synopsis of infrageneric categories in
Celmisia (Astereae-Compositae). – New Zealand J. Bot. 7: 389-418.
Given DR. 1973. Damnamenia gen. nov.
A new subantarctic genus allied to Celmisia Cass.
(Astereae-Compositae). – New Zealand J. Bot. 11: 785-796.
Given DR. 1980. A taxonomic revision of
Celmisia coriacea (Forst. f.) Hook. f. and its immediate allies
(Astereae-Compositae). – New Zealand J. Bot. 18: 127-140.
Given DR. 1984. A taxonomic revision of
Celmisia subgenus Pelliculatae section Petiolatae
(Compositae-Astereae). – New Zealand J. Bot. 22: 139-158.
Givnish TJ, Sytsma KJ, Smith JF, Hahn WJ.
1994. Thornlike prickles and heterophylly in Cyanea: adaptations to
extinct avian browsers in Hawaii? – Proc. Natl. Acad. Sci. U.S.A. 91:
2810-2814.
Givnish TJ, Sytsma KJ, Smith JF, Hahn WJ.
1995. Molecular evolution, adaptive radiation, and geographic speciation in
Cyanea (Campanulaceae,
Lobelioideae). – In: Wagner WL, Funk VA (eds), Hawaiian biogeography:
evolution on a hotspot archipelago, Smithsonian Institution Press, Washington,
D.C., pp. 288-337.
Givnish TJ, Millam KC, Mast AR, Paterson TB,
Theim TJ, Hipp AL, Henss JM, Smith JF, Wood KR, Sytsma KJ. 2009. Origin,
adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae). – Proc. Roy.
Soc. 276, B: 407-416.
Gleason HA. 1924. Studies of the flora of
northern South America I. Centropogon, Section
Burmeisteroides. – Bull. Torrey Bot. Club 51: 443-448.
Glenny D. 2009. A revision of the genus
Forstera (Stylidiaceae). – New Zealand J.
Bot. 47: 285-315.
Gnecco S, Bartulin J, Becerra J, Marticorena
C. 1989. n-Alkanes from Chilean Euphorbiaceae
and Compositae species. – Phytochemistry 28: 1254-1256.
Goertzen LR, Francisco-Ortega J,
Santos-Guerra A, Mower JP, Linder CR, Jansen RK. 2002. Molecular systematics of
the Asteriscus alliance (Asteraceae: Inuleae) II: combined
nuclear and chloroplast data. – Syst. Bot. 27: 815-823.
Goertzen LR, Cannone JJ, Gutell RR, Jansen
RK. 2003. ITS secondary structure derived from comparative analysis:
implications for a sequence alignment and phylogeny of Asteraceae. – Mol. Phylogen. Evol.
29: 216-234.
Gomez F, Quijano L, Calderon JS, Perales A,
Rios T. 1983. 11,13-dihydroeucannabolide, a new heliangolide from Stevia
monardaefolia. – Phytochemistry 22: 197-199.
Gomez F, Quijano L, Calderon JS, Rios T.
1983. 3-angeloyoxy-2-hydroxycativic acid, a new diterpene from Brickellia
paniculata. – Phytochemistry 22: 1292-1293.
Gomiz F, Llamas F. 2005. A new species of
Senecio (Asteraceae) from
North Africa. – Syst. Bot. 30: 899-903.
Gomürgen AN, Dogan C, Ozmen E, Aytac Z.
2009. Chromosome number, karyotype and pollen morphology of the rare endemic
Centaurea goeksunensis. – Nord. J. Bot. 27: 120-124.
Gonzál CB, Brion JM. 1985. Carpología del
género Anthemis L. en la Peninsula Ibérica e Islas Baleares. –
Coll. Bot. 16: 77-87.
González AG. 1977. Lactuceae – chemical
review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae 2, Academic Press, London, pp. 1081-1095.
González AG, Arteaga JM, Breton JL, Fraga
BM. 1977. Five new labdane diterpenes from Eupatorium jahnii. –
Phytochemistry 16: 107-110.
González-Pérez MA, Sosa PA,
González-González EA, Bañares A, Marrero M, Cargue E, Polifrone M. 2008.
Gnaphalium teydeum and Gnaphalium luteo-album: two taxa of
the Canary Islands with different genetic histories. – Plant Syst. Evol. 276:
39-49.
González Sierra G, Pérez Morales C, Penas
Merion A, Rivas-Martínez S. 1992. Revisión taxonomica de las especies
ibericas del género Onopordum L. – Candollea 47: 181-213.
Good R. 1924. New South African Campanulaceae. – J. Bot. 62:
48-50.
Good R. 1925. On the geographical
distribution of the Stylidiaceae. – New Phytol. 24:
225-240.
Gottlieb LD. 1981. Gene number of species of
Asteraceae that have different
chromosome number. – Proc. Natl. Acad. Sci. U.S.A. 78: 3726-3729.
Gottschlich G. 2009. Die Gattung
Hieracium L. (Compositae) in der Region Abruzzen (Italien). –
Stapfia 89: 1-328.
Gowik U, Bräutigam A, Weber KL, Weber APM,
Westhoff P. 2011. Evolution of C4 photosynthesis in the genus
Flaveria: How many and which genes does it take to make C4?
– Plant Cell 23: 2087-2105.
Graham A. 1996. A contribution to the
geological history of the Compositae. – In: Hind DJN, Beentje HJ (eds),
Compositae: systematics. Proceedings of the International Compositae
Conference, Kew, 1994, vol. 1, Royal Botanic Gardens, Kew, pp. 123-140.
Grant JR. 1994. Xerxes, a new name
to replace Alcantara (Asteraceae: Vernonieae). – Nord. J.
Bot. 14: 287-288.
Grashoff J. 1974. Novelties in
Stevia (Compositae: Eupatorieae). – Brittonia 26: 347-384.
Grashoff J. 1975. Metastevia
(Compositae: Eupatieae): a new genus from Mexico. – Brittonia27: 69-73.
Grau J. 1970. Die Gattung
Polyarrhena Cass. (Asteraceae-Astereae). – Mitt. Bot.
Staatssamml. München 7: 347-368.
Grau J. 1973. Revision der Gattung
Felicia (Asteraceae). –
Mitt. Bot. Staatssamml. München 9: 195-705.
Grau J. 1975. Podocoma und
Vittadinia – zwei vermeintlich bikontinentale Gattungen. – Mitt.
Bot. Staatssamml. München 12: 181-194.
Grau J. 1976. Chromosomenzahlen von
südamerikanischen Haplopappus-Arten. – Mitt. Bot. Staatssamml.
München 12: 403-410.
Grau J. 1977a. Poecilolepis – eine
neue Gattung der Asteraceae-Astereae. – Mitt. Bot.
Staatssamml. München 13: 243-254.
Grau J. 1977b. Astereae – systematic
review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae, Academic Press, London, pp. 539-565.
Grau J. 1980. Die Testa der Mutisieae und
ihre systematische Bedeutung. – Mitt. Bot. Staatssamml. München 16:
269-332.
Grau J. 1981. Zur Zytologie und
systematischen Stellung von Haplopappus pectinatus Phil. – Mitt.
Bot. Staatssamml. München 17: 527-536.
Grau J, Hopf H. 1985. Das Endosperm der
Compositae. – Bot. Jahrb. Syst. 107: 251-268.
Gray A. 1852. Characters of some
south-western Australian Compositae, principally of the subtribe Gnaphalieae.
– Hooker’s J. Bot. Kew Gard. Misc. 4: 225-232.
Gray M, Given DR. 1999. New species and a new
combination in Australian Celmisia (Asteraceae-Astereae). – Aust. Syst.
Bot. 12: 201-206.
Greene EL. 1904. Affinities of the
Cichoriaceae. – Leafl. Bot. Observ. Crit. 1: 59-63.
Greenman JM. 1903. Monographie der nord- und
centralamerikanischen Arten der Gattung Senecio. – Engl. Bot. Jahrb.
Syst. 32: 1-33.
Greenman JM. 1916. Monograph of the North and
Central American species of the genus Senecio II. – Ann. Missouri
Bot. Gard. 3: 85-188.
Greger H. 1975. Laubblatt-Flavonoide und
Systematik bei Matricaria und Tripleurospermum (Asteraceae-Anthemideae). – Plant
Syst. Evol. 124: 35-55.
Greger H. 1977a. Anthemideae – chemical
review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae 2, Academic Press, London, pp. 899-941.
Greger H. 1977b. Comparative phytochemistry
and systematics of Anacyclus (Asteraceae-Anthemideae). – Biochem.
Syst. Ecol. 6: 11-17.
Greger H, Hofer O, Robien W. 1983. Types of
sesquiterpen-coumarin ethers from Achillea ochroleuca and
Artemisia tripartita. – Phytochemistry 22: 1997-2003.
Grehan JR. 2007. A bfeif look at Pacific
biogeography: the trans-oceanic travels of Microseris (Angiosperms: Asteraceae). – In: Ebach MC,
Tangney RS (eds), Biogeography in a changing world, Syst. Assoc. Spec. Vol.
Ser. 70, pp. 83-94.
Greiner R, Vogt R, Oberprieler C. 2012.
Phylogenetic studies in the polyploid complex of the genus
Leucanthemum Mill. (Compositae, Anthemideae) based on cpDNA sequence
variation. – Plant Syst. Evol. 298: 1407-1414.
Greuter W. 1973. Monographie der Gattung
Ptilostemon (Compositae). – Boissiera 22.
Greuter W. 1997. Save Asteriscus,
sink Nauplius (Compositae). – Flora Mediterranea 7: 41-48.
Greuter W. 2003a. The Euro+Med treatment of
Astereae (Compositae) – generic concepts and required new names. –
Willdenowia 33: 45-47.
Greuter W. 2003b. The Euro+Med treatment of
Cardueae (Compositae) – generic concepts and required new names. –
Willdenowia 33: 49-61.
Greuter W. 2003c. The Euro+Med treatment of
Cichorieae (Compositae) – generic concepts and required new names. –
Willdenowia 33: 229-238.
Greuter W. 2003d. The Euro+Med treatment of
Gnaphalieae and Inuleae (Compositae) – generic concepts and required new
names. – Willdenowia 33: 239-244.
Greuter W. 2003e. The Euro+Med treatment of
Senecioneae and the minor Compositae tribes – generic concepts and required
new names, with an addendum to Cardueae. – Willdenowia 33: 245-250.
Greuter W, Wagenitz G, Agababian M, Hellwig
FH. 2001. Proposal to conserve the name Centaurea (Compositae) with a
conserved type. – Taxon 50: 1201-1205.
Greuter W, Oberprieler C, Vogt R. 2003. The
Euro+Med treatment of Anthemideae (Compositae) – generic concepts and
required new names. – Willdenowia 33: 37-43.
Greuter W, Aghababian M, Wagenitz G. 2005.
Proposals top conserve the names Bellidiastrum, Berkheya,
Euryops, Notobasis, Picnomon and Urospermum
(Compositae) against six generic names of Vaillant. – Taxon 54: 196-198.
Greuter W, Aghababian M, Wagenitz G. 2005.
Vaillant on Compositae – systematic concepts and nomenclatural impact. –
Taxon 54: 149-174.
Greuter W, Gutermann W, Talavera S. 2006. A
preliminary conspectus of Scorzoneroides (Compositae, Cichorieae) with
validation of the required new names. – Willdenowia 36: 689-692.
Grey-Wilson C. 1990. A survey of
Codonopsis in cultivation. – The Plantsman 12: 64-99.
Grey-Wilson C. 1995. Codonopsis
convolvulacea and its allies. – The New Plantsman 2: 213-225.
Grierson AJC. 1967. The genus
Psychrogeton (Compositae). – Notes Roy. Bot. Gard. Edinb. 27:
101-147.
Grossi MA. 2011. Revisión sistemática,
análisis cladistico y biogeografía del género Stomatanthes R. M.
King & H. Rob. (Asteraceae,
Eupatorieae). – PhD diss., Facultad de Ciencias Naturales y Museo,
Universidad Nacional de La Plata, La Plata, Argentina.
Grossi MA, Katinas L. 2013. A new
circumscription of the genus Stomatanthes (Asteraceae, Eupatorieae). – Syst.
Bot. 38: 830-849.
Grossi MA, Katinas L, Nakajima JN. 2013.
Criscianthus, a new genus of Eupatorieae (Asteraceae) with a key to members of
the tribe in Africa. – Phytotaxa 141: 25-39.
Grossi MA, Gutiérrez DG, Berrueta PC,
Martínez JJ. 2011. Acanthostyles (Asteraceae, Eupatorieae): a revision
with a multivariate analysis. – Aust. Syst. Bot. 24: 87-103.
Gruenstaeudl M, Urtubey E, Jansen RK, Samuel
R, Barfuss MHJ, Stuessy TF. 2009. Phylogeny of Barnadesioideae (Asteraceae) inferred from DNA
sequence data and morphology. – Mol. Phylogen. Evol. 51: 572-587.
Gruenstaeudl M, Santos-Guerra A, Jansen RK.
2013. Phylogenetic analyses of Tolpis Adans. (Asteraceae) reveal patterns of
adaptive radiation, multiple colonization and interspecific hybridization. –
Cladistics 29: 416-434.
Gudkova IY, Borshchenko GP. 1991. The
serological study of the Campanulaceae. The phylogenetic
relations in the tribe Phyteumateae. – Bot. Žurn. 76: 809-817. [In Russian
with English summary]
Guinochet M. 1957. Contribution à l’étude
caryologique du genre Centaurea L. sens. lat. – Bull. Soc. Hist.
Nat. Afr. Nord 48: 282-300.
Guinochet M, Foissac J. 1962. Sur les
caryotypes de quelques espèces du genre Centaurea L. et leur
signification taxonomique. – Rev. Cytol. Biol. Vég. 25: 373-389.
Guo Y-P, Ehrendorfer F, Samuel R. 2004.
Phylogeny and systematics of Achillea (Asteraceae-Anthemideae) inferred from
nrITS and plastid trnL-F DNA sequences. – Taxon 53: 657-672.
Guo Y-P, Saukel J, Mittermayr R, Ehrendorfer
F. 2005. AFLP analyses demonstrate genetic divergence, hybridization, and
multiple polyploidization in the evolution of Achillea (Asteraceae-Anthemideae). – New
Phytologist 166: 273-289.
Gupta S, Mukherjee A, Mondal M. 2000. A
review of the Menyanthaceae
Dumortier in India. – Acta Bot. Hung. 42: 119-137.
Gustafsson M, Snogerup S. 1973.
Scorzonera scyria, a new chasmophytic species from Greece. – Bot.
Not. 125: 323-328.
Gustafsson MHG. 1995. Petal venation in the
Asterales and related orders. – Bot. J. Linn. Soc. 118: 1-18.
Gustafsson MHG. 1996a. Phylogenetic studies
in the Asterales sensu lato. – Ph.D. diss., University of Uppsala, Sweden.
Gustafsson MHG. 1996b. Phylogenetic
hypotheses for Asteraceae
relationships. – In: Hind DJN, Beentje HJ (eds), Compositae: systematics.
Proceedings of the International Compositae Conference, Kew, 1994, vol. 1,
Royal Botanic Gardens, Kew, pp. 9-19.
Gustafsson MHG. 2006 [2007]. Carpodetaceae.
– In: Kubitzki K, Kadereit JW, Jeffrey C (eds), The families and genera of
vascular plants VIII. Flowering plants. Eudicots. Asterales, Springer, Berlin,
Heidelberg, New York, pp. 57-60.
Gustafsson MHG, Bremer K. 1995. Morphology
and phylogenetic interrelationships of the Asteraceae, Calyceraceae, Campanulaceae, Goodeniaceae, and related familes
(Asterales). – Amer. J. Bot. 82: 250-265.
Gustafsson MHG, Bremer K. 1997. The
circumscription and systematic position of Carpodetaceae. – Aust. Syst. Bot.
10: 855-862.
Gustafsson MHG, Backlund A, Bremer B. 1996.
Phylogeny of the Asterales sensu lato based on rbcL sequences with
particular reference to the Goodeniaceae. – Plant Syst. Evol.
199: 217-242.
Gustafsson MHG, Grafström E, Nilsson S.
1997. Pollen morphology of the Goodeniaceae and comparisons with
related families. – Grana 36: 185-207.
Gustafsson MHG, Pepper ASR, Albert VA,
Källersjö M. 2001. Molecular phylogeny of the Barnadesioideae. – Nord. J.
Bot. 21: 149-160.
Gustafsson Å. 1932. Zytologische und
experimentelle Studien in der Gattung Taraxacum. – Hereditas 16:
41-62.
Gutiérrez DG. 2010. Inkaliabum, a
new Andean genus of Liabeae (Asteraceae) from Peru. – Bol. Soc.
Argent. Bot. 45: 363-372.
Gutiérrez-Larruscain D, Santos-Vicente M,
Anderberg AA, Rico E, Martínez-Ortega MM. 2018. Phylogeny of the
Inula group (Asteraceae:
Inuleae): evidence from nuclear and plastid genomes and a recircumscription of
Pentanema. – Taxon 67: 149-164.
Gutterman Y, Ginott S. 1994. Long-term
protected ‘seed bank’ in dry inflorescences of Asteriscus
pygmaeus: achene dispersal mechanism and germination. – J. Arid
Environm. 26: 149-163.
Haberle RC. 1998. Phylogenetic systematics of
Pseudonemacladus and the North American cyphioids (Campanulaceae sensu
lato). – M.Sc. thesis, Northern Arizona University.
Haberle RC, Fourcade HM, Boore JL, Jansen RK.
2008. Extensive rearrangements in the chloroplast genome of Trachelium
caeruleum are associated with repeats and tRNA genes. – J. Mol. Evol.
66: 350-361.
Haberle RC, Dang A, Lee T, Peñaflor C,
Cortes-Burns H, Oestreich A, Raubeson L, Cellinese N, Edwards EJ, Kim S-T,
Eddie WMM, Jansen RK. 2009. Taxonomic and biogeographic implications of a
phylogenetic analysis of the Campanulaceae based on three
chloroplast genes. – Taxon 58: 715-734.
Haesler I. 1967. Chromosomenzahlen aus der
Gattung Ursinia. – Mitt. Bot. Staatssamml. München 6: 531-539.
Häffner E. 2000. On the phylogeny of the
subtribe Carduinae (tribe Cardueae, Compositae). – Englera 21: 1-209.
Häffner E, Hellwig F. 1999. Phylogeny of the
tribe Cardueae (Compositae) with emphasis on subtribe Carduinae: an analysis
based on ITS sequence data. – Willdenowia 29: 27-39.
Hair JB. 1962. Basic chromosome numbers in
Cotula. – Chromosome Inform. Serv. 3: 41-42.
Hall HM. 1928. The genus
Haplopappus. A phylogenetic study in the Compositae. – Carnegie
Institution of Washington, Washington, D.C.
Hall HM, Clements FC. 1923. The phylogenetic
method in taxonomy. The North American species of Artemisia,
Chrysothamnus, and Atriplex. – Publ. Carnegie Inst.
Washington 326: 1-355.
Halliday P. 1984. The genus Kleinia
(Compositae) in Arabia. – Kew Bull. 39: 817-827.
Halliday P. 1986. The genus Kleinia
(Compositae) in North Africa and the Canary Islands. – Kew Bull. 41:
279-285.
Halliday P. 1988. Noteworthy species of
Kleinia. – Hooker’s Icones Plant. 39(4): 1-135, Tab. 3876-3900.
Halvorsen T, Borgen L. 1986. The perennial
Macaronesian species of Bubonium (Compositae-Inuleae). –
Sommerfeltia 3: 1-103.
Hamel J. 1953. Contribution à l’étude
cytotaxinomique des Saxifragacées. – Rev. Cytol. Biol. Vég. 14: 113-311.
Hamzaoglu E, Budak U. 2009. Centaurea
aksoyi sp. nov. (Asteraceae:
Cardueae) from Turkey and a contribution to the sectional taxonomy. – Nord.
J. Bot. 27: 16-20.
Hand R, Hadjikyriakou G. 2009. Cynara
makrisii (Asteraceae,
Cardueae), a new artichoke species in Cyprus. – Willdenowia 39: 77-81.
Handel-Mazzetti H von. 1907. Monographie der
Gattung Taraxacum. – Leipzig.
Hänsel R, Schulz H, Leuckert C. 1964. Das
Lignanglykosid Arctiin als chemotaxonomisches Merkmal in der Familie der
Compositae. – Zeitschr. Naturf. 19b: 727-734.
Hansen HV. 1985a. Notes on Gerbera
sect. Pseudoseris (Compositae-Mutisieae). – Nord. J. Bot. 5:
451-453.
Hansen HV. 1985b. A taxonomic revision of the
genus Gerbera (Compositae, Mutisieae) sections Gerbera,
Parva, Piloselloides (in Africa), and Lasiopus. –
Opera Bot. 78: 1-36.
Hansen HV. 1985c. A taxonomic revision of the
genus Perdicium (Compositae-Mutisieae). – Nord. J. Bot. 5:
543-546.
Hansen HV. 1988. A taxonomic revision of the
genera Gerbera sect. Isanthus, Leibnitzia (in Asia),
and Uechtritzia (Compositae, Mutisieae). – Nord. J. Bot. 8:
61-76.
Hansen HV. 1990. Phylogenetic studies in the
Gerbera complex (Compositae, tribe Mutisieae, subtribe Mutisiinae).
– Nord. J. Bot. 9: 469-485.
Hansen HV. 1991a. Phylogenetic studies in
Compositae tribe Mutisieae. – Opera Bot. 109: 1-50.
Hansen HV. 1991b. SEM-studies and general
comments on pollen in tribe Mutisieae (Compositae) sensu Cabrera. – Nord. J.
Bot. 10: 607-623.
Hansen HV. 1992. Studies in the Calyceraceae with a discussion of
its relationship to Compositae. – Nord. J. Bot. 12: 63-75.
Hansen HV. 1997. Studies in the Goodeniaceae and the Brunoniaceae
with a discussion of their relationships to Asteraceae and Calyceraceae. – Nord. J. Bot. 17:
495-510.
Hansen HV, Christensen KI. 2009. The common
chamomile and the scentless mayweed revisited. – Taxon 58: 261-264.
Hansen HV, Hjerting JP. 1996. Observations on
chromosome numbers and biosystematics in Dahlia (Asteraceae, Heliantheae) with an
account on the identity of D. pinnata, D. rosea, and D.
coccinea. – Nord. J. Bot. 16: 445-455.
Haque MZ, Godward MBE. 1984. New records of
the carpopodium in Compositae and its taxonomic use. – Bot. J. Linn. Soc. 89:
321-340.
Harborne JB, Heywood VH, Saleh NAM. 1970.
Chemosystematics of the Compositae: flavonoid patterns in the
Chrysanthemum complex of the tribe Anthemideae. – Phytochemistry 9:
2011-2017.
Harker M, Jimenez-Reyes N. 2002.
Verbesina barrancae (Compositae, Heliantheae), a new species from
Jalisco, Mexico. – Brittonia 54: 181-189.
Harling G. 1950. Embryological studies in the
Compositae I. Anthemideae-Anthemidinae. – Acta Horti Berg. 15: 135-168.
Harling G. 1951a. Embryological studies in
the Compositae II. Anthemideae-Chrysantheminae. – Acta Horti Berg. 16:
1-56.
Harling G. 1951b. Embryological studies in
the Compositae III. Astereae. – Acta Horti Berg. 16: 73-120.
Harling G. 1960. Further embryological and
taxonomical studies in Anthemis L. and some related genera. – Svensk
Bot. Tidskr. 54: 572-590.
Harling G. 1962. On some Compositae endemic
to the Galápagos Islands. – Acta Horti Berg. 20: 63-120.
Harling G. 1991. 190(10).
Compositae-Mutisieae. – In: Harling G, Andersson L (eds), Flora of Ecuador
42, Nord. J. Bot., Copenhagen, pp. 1-105.
Harling G. 1995. The genus Jungia L.
fil. (Compositae-Mutisieae). – Acta Regiae Soc. Sci. Litt. Gothob. Bot. 4:
1-133.
Harms H. 1898. Cornaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien III(8), W. Engelmann,
Leipzig, pp. 250-270.
Harms VL. 1965. Cytogenetic evidence
supporting the merger of Heterotheca and Chrysopsis
(Compositae). – Brittonia 17: 11-16.
Harms VL. 1974a. A preliminary conspectus of
Heterotheca section Chrysopsis (Compositae). – Castanea 39:
155-165.
Harms VL. 1974b. Chromosome numbers in
Heterotheca including Chrysopsis (Compositae: Astereae) with
phylogenetic interpretations. – Brittonia 26: 61-69.
Haro-Carrión X, Robinson H. 2008. A review
of the genus Critoniopsis in Ecuador (Vernonieae: Asteraceae). – Proc. Biol. Soc.
Washington 121: 1-18.
Harriman NA. 1998. Proposal to conserve the
name Bidens (Asteraceae)
with a conserved gender. – Taxon 47: 485-486.
Harris EM. 1991. Comparative inflorescence
and floral development in the Compositae. – Ph.D. diss., Louisiana State
University, Baton Rouge, Louisiana.
Harris EM. 1995. Inflorescence and floral
ontogeny in Asteraceae: a
synthesis of historical and current concepts. – Bot. Rev. 61: 93-275.
Harris EM. 1999. Capitula in the Asteridae: a
widespread and varied phenomenon. – Bot. Rev. 65: 348-369.
Harris EM, Tucker SC, Urbatsch LE. 1991.
Floral initiation and early development in Erigeron philadelphicus (Asteraceae). – Amer. J. Bot. 78:
108-121.
Harris T. 2013. A treatment of the African
genus Schistostephium (Compositae: Anthemideae). – Kew Bull. 68:
107-120.
Hart CR. 1979. The systematics of the
Bidens ferulaefolia complex (Compositae). – Syst. Bot. 4:
130-147.
Hartman RL. 1976. A conspectus of
Machaeranthera (Compositae: Astereae) and a biosystematic study of the
section Blepharodon. – Ph.D. diss., University of Texas, Austin,
Texas.
Hartman RL. 1990. A conspectus of
Machaeranthera (Asteraceae: Astereae). – Phytologia
68: 439-465.
Hartman RL, Stuessy TF. 1983. Revision of
Otopappus (Compositae, Heliantheae). – Syst. Bot. 8: 185-210.
Haslett BG, Gleaves T, Boulter D. 1977.
N-terminal amino acid sequences of plastocyanins from various members of the
Compositae. – Phytochemistry 16: 363-365.
Hauman L. 1934. Les “Lobelia”
géants des montagnes du Congo Belge. – Mém. 8º Inst. Roy. Col. Belg. Sect.
Sci. Nat. 2: 1-52.
Heads M. 1998. Biodiversity in the New
Zealand divaricating tree daises: Olearia sect. nov. (Compositae). –
Bot. J. Linn. Soc. 127: 239-285. – Erratum: 131 (1999): 101.
Heads M. 1999. Vicariance biogeography and
terrane tectonics in the South Pacific: analysis of the genus
Abrotanella (Compositae). – Biol. J. Linn. Soc. 67: 391-432.
Heads M. 2010. The endemic plant families and
the palms of New Caledonia: a biogeographical analysis. – J. Biogeogr. 37:
1239-1250.
Heath PV. 1997. Three new gener names in Asteraceae 1. – Calyx 5: 136.
Heath PV. 1999. Three new generic names in Asteraceae 2. – Calyx 6: 54-55.
Hedberg O. 1961. Monograph of the genus
Canarina L. (Campanulaceae). – Svensk Bot.
Tidskr. 55: 16-62.
Heenan PH. 1997. Selliera
rotundifolia (Goodeniaceae), a new, round-leaved,
species from New Zealand. – New Zealand J. Bot. 35: 133-138.
Hegnauer R. 1977. The chemistry of the
Compositae. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae, Academic Press, London, pp. 283-335.
Heiden G, Pirani JR. 2012. A synopsis and
notes for Baccharis subgen. Tarchonanthoides (Asteraceae: Astereae). – Phytotaxa
60: 41-49.
Heidenhain B. 1952. Über die Blütenstände
der Campanulaceen. – Akad. Wiss. Abh. Math.-Naturwiss. Kl. 9: 621-650.
Heim G. 1984. Die Compositen-Gattungen
Conyza, Blumea und Pluchea auf den Kapverdischen
Inseln (Phanerogamae: Asteraceae).
– Courier Forschungsinst. Senckenberg 68: 143-178.
Heimerl A. 1884. Monographia sectionis
‘Ptarmica’ Achilleae generis. Die Arten, Unterarten, Varietäten
uynd Hybriden der Section Ptarmica des Genus Achillea. –
Denkschr. K. Akad. Wiss. Wien, Math.-Naturwiss. Kl. 48: 113-192.
Heiser CB. 1945. A revision of the genus
Schkuhria. – Ann. Missouri Bot. Gard. 32: 265-278.
Heiser CB. 1957. A revision of the South
American species of Helianthus. – Brittonia 8: 283-295.
Heiser CB. 1963. Artificial intergeneric
hybrids of Helianthus and Viguiera. – Madroño 17:
118-127.
Heiser CB, Martin C, Smith DM. 1962. Species
crosses in Helianthus I. Diploid species. – Brittonia 14:
137-147.
Helariutta Y, Kotilainen M, Elomaa P,
Kalkkinen N, Bremer K, Teeri TH, Albert VA. 1996. Duplication and functional
divergence in the chalcone synthase gene family of Asteraceae: evolution with substrate
change and catalytic simplification. – Proc. Natl. Acad. Sci. U.S.A. 93:
9033-9038.
Helenurm K, Ganders FR. 1985. Adaptive
radiation and genetic differentiation in Hawaiian Bidens. –
Evolution 39: 753-765.
Hellwig FH. 1990. Die Gattung
Baccharis L. (Compositae-Astereae) in Chile. – Mitt. Bot.
Staatssamml. München 29: 1-456.
Hellwig FH. 1993. The genera
Pingraea Cass. and Neomolina Hellwig (Compositae-Astereae).
– Candollea 48: 203-219.
Hellwig FH. 1994. Chromosomenzahlen aus der
Tribus Cardueae (Compositae). – Willdenowia 24: 219-248.
Hellwig FH. 1996a. Untersuchungen zur
Phylogenie der Cardueae-Centaureinae (Compositae) unter Verwendung molekularer
und morphologisch-anatomischer Merkmale. – Habilitationsschrift, Universität
Göttingen.
Hellwig FH. 1996b. Taxonomy and evolution of
Baccharidinae (Compositae). – In: Hind DJN, Beentje HJ (eds), Compositae:
systematics. Proceedings of the International Compositae Conference, Kew, 1994,
vol. 1, Royal Botanic Gardens, Kew, pp. 575-590.
Hellwig FH. 2004. Centaureinae (Asteraceae) in the Mediterranean –
History of ecogeographical radiation. – Plant Syst. Evol. 246: 137-162.
Hellwig FH. 2006 [2007]. Calyceraceae. – In: Kubitzki K,
Kadereit JW, Jeffrey C (eds), The families and genera of vascular plants VIII.
Flowering plants. Eudicots. Asterales, Springer, Berlin, Heidelberg, New York,
pp. 19-25.
Hellwig FH, Oberprieler C, Vogt R, Wagenitz
G. 1994. Chromosome numbers of North African phanerogams III. Some counts in
Centaurea (Compositae, Cardueae). – Willdenowia 24: 249-254.
Hendry GAF. 1996. Fructan and the ecology and
evolution of the Compositae. – In: Caligari PDS, Hind DJN (eds), Compositae:
biology and utilization. Proceedings of the International Compositae
Conference, Kew, 1994, vol. 2, Royal Botanic Gardens, Kew, pp. 121-128.
Henrickson J. 1976.
Marshalljohnstonia, a new genus (Asteraceae) with a rosett-shrub
growth habit from Mexico. – Syst. Bot. 1: 169-180.
Herborg J. 1987. Die Variabilität und
Sippenabgrenzung in der Senecio nemorensis-Gruppe (Compositae) im
europäischen Teilareal. – Diss. Bot. 107: 1-262.
Herman PPJ. 1999. Synopsis of the genus
Rennera Merxm. (Asteraceae, Anthemideae) with the
description of a new species from South Africa. – Bot. J. Linn. Soc. 129:
367-377.
Herman PPJ. 2013. Cypsela morphology in the
genus Nolletia (Asteraceae, Astereae) and a revision
of the genus. – Phytotaxa 122: 1-44.
Herman PPJ, Zinnecker-Wiegand U. 2016. A
taxonomic revision of the genus Mairia (Asteraceae, Astereae) in South
Africa. – South Afr. J. Bot. 105: 45-60.
Hershkovitz MA, Arroyo MTK, Bell C, Hinojosa
LF. 2006. Phylogeny of Chaetanthera (Asteraceae: Mutisieae) reveals both
ancient and recent origins of the high elevation lineages. – Mol. Phylogen.
Evol. 41: 594-605.
Herz W. 1977. Sesquiterpene lactones in the
Compositae. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae, vol. 2, Academic Press, London, pp. 337-357.
Herz W, Högenauer G. 1962. Ivalin, a new
sesquiterpene lactone. – J. Org. Chem. 27: 905-910.
Herz W, Govindan SV. 1979. Tetracyclic
analogues of the rosane lactones from Eupatorium album. – J. Org.
Chem. 44: 2999-3003.
Herz W, Kulanthaivel P. 1982. New flavones
from Eupatorium leucolepis. – Phytochemistry 21: 2363-2366.
Herz W, Kulanthaivel P. 1983. Eudesmanolides
and ent-pimaranes from Liatris laevigata. – Phytochemistry
22: 715-720.
Herz W, Ramakrishnan G. 1978. δ-Lactones of
polyhydroxy-C26 acids in Eupatorium pilosum. –
Phytochemistry 17: 1327-1332.
Herz W, Sharma RP. 1975. A
trans-1,2-cis-4,5-germacranolide and other new germacranolides from
Tithonia species. – J. Org. Chem. 40: 3118-3123.
Herz W, Sharma RP. 1976. New hydroxylated
ent-kauranoic acids from Eupatorium album. – J. Org. Chem.
41: 1021-1026.
Herz W, Gibaja S, Bhat SV, Srinivasan A.
1972. Dihydroflavonols and other flavonoids of Eupatorium species. –
Phytochemistry 11: 2859-2863.
Herz W, Srinivasan A, Kalyanaraman PS. 1975.
Mikanokryptin, a new guaianolide from Mikania. – Phytochemistry 14:
233-237.
Herz W, Kalyanaraman PS, Ramakrishnan G.
1977. Sesquiterpene lactones of Eupatorium perfoliatum. – J. Org.
Chem. 42: 2264-2271.
Herz W, Kumar N, Blount JF. 1980. A
thiol-containing ester side chain in a sesquiterpene lactone from
Eupatorium mikanioides. – J. Org. Chem. 45: 489-493.
Heslop-Harrison J. 1969. An
acetolysis-resistant membrane investing tapetum and sporogenous tissue in the
anthers of certain Compositae. – Can. J. Bot. 47: 541-542.
Hess R. 1938. Vergleichende Untersuchungen
über die Zwillinghaare der Compositen. – Bot. Jahrb. Syst. 68: 435-496.
Heusden AW van, Bachmann K. 1992a. Genotype
relationships in Microseris elegans (Asteraceae, Lactuceae) revealed by
DNA amplification from arbitrary primers (RAPDs). – Plant Syst. Evol. 179:
221-233.
Heusden AW van, Bachmann K. 1992b. Genetic
differentiation of Microseris pygmaea (Asteraceae, Lactuceae) studied with
DNA amplification from arbitrary primers (RAPDs). – Acta Bot. Neerl. 41:
385-395.
Heyn CC, Dagan O, Nachman B. 1974. The annual
Calendula species: taxonomy and relationships. – Israel J. Bot. 23:
169-201.
Heywood VH, Humphries CJ. 1977. Anthemideae
– systematic review. – In: Heywood VH, Harborne JB, Turner BL (eds), The
biology and chemistry of the Compositae 2, Academic Press, London, pp.
851-898.
Heywood VH, Harborne JB, Turner BL (eds).
1977a. The biology and chemistry of the Compositae. – Academic Press,
London.
Heywood VH, Harborne JB, Turner BL. 1977b. An
overture to the Compositae. – In: Heywood VH, Harborne JB, Turner BL (eds),
The biology and chemistry of the Compositae, Academic Press, London, pp.
1-20.
Hidalgo O, Garcia-Jacas N, Garnatje T,
Susanna A. 2006. Phylogeny of Rhaponticum (Asteraceae, Cardueae-Centaureinae)
and related genera inferred from nuclear and chloroplast DNA sequence data:
taxonomic and biogeographic implications. – Ann. Bot. 97: 705-714.
Hidalgo O, Garcia-Jacas N, Garnatje T,
Susanna A, Siljak-Yakovlev S. 2007. Karyological evolution in
Rhaponticum Vaill. (Asteraceae, Cardueae) and related
genera. – Bot. J. Linn. Soc. 153: 193-201.
Hidalgo O, Garcia-Jacas N. Garnatje T,
Romashchenko K, Susanna A, Siljak-Yakovlev S. 2008. Extreme environmental
conditions and phylogenetic inheritance: systematics of Myopordon and
Oligochaeta (Asteraceae,
Cardueae-Centaureinae). – Taxon 57: 769-778.
Hideux M, Ferguson IK. 1976. The
stereostructure of the exine and its evolutionary significance in Saxifragaceae
sensu lato. – In: Ferguson IK, Muller J (eds), The evolutionary significance
of the exine, Linn. Soc. Symposium, No. 1, Academic Press, London, New York,
pp. 327-377.
Hiern WP. 1898. Two new genera of the
Compositae. – J. Bot. 36: 289-291.
Hikichi M, Furuya T. 1974. Syneilesine, a new
pyrrolizidine alkaloid from Syneilesis palmata. – Tetrahedron Lett.
1974: 3657-3660.
Hilliard OM. 1977. Compositae in Natal. –
Pietermaritzburg, Republic of South Africa.
Hilliard OM, Burtt BL. 1973. Notes on some
plants of southern Africa chiefly from Natal III. – Notes Roy. Bot. Gard.
Edinb. 32: 303-387.
Hilliard OM, Burtt BL. 1975. Notes on some
plants of southern Africa chiefly from Natal IV. – Notes Roy. Bot. Gard.
Edinb. 34: 73-100.
Hilliard OM, Burtt BL. 1981. Some generic
concepts in Compositae-Gnaphaliinae. – Bot. J. Linn. Soc. 82: 181-232.
Hilpold A, Vilatersana R, Susanna A, Meseguer
AS, Boršić, Constantinidis T, Filigheddu R, Romaschenko K, Suárez-Santiago
VN, Tugay O, Uysal T, Pfeil BE, Garcia-Jacas N. 2014. Phylogeny of the
Centaurea group (Centaurea, Compositae) – geography is a
better predictor than morphology. – Molec. Phylogen. Evol. 77: 195-215.
Hils MH. 1985. Comparative anatomy and
systematics of twelve woody Australasian genera of the Saxifragaceae.
– Ph.D. diss., University of Florida, Gainesville, Florida.
Himmelreich S, Källersjö M, Eldenäs P,
Oberprieler C. 2008. Phylogeny of southern hemisphere Compositae-Anthemideae
based on nrDNA ITS and cpDNA ndhF sequence information. – Plant
Syst. Evol. 272: 131-153.
Himmelreich S, Breitwieser I, Oberprieler C.
2014. Phylogenetic relationships in the extreme polyploidy complex of the New
Zealand genus Leptinella (Compositae: Anthemideae) based on AFLP data.
– Taxon 63: 883-898.
Hind DJN. 1993a. Notes on the Compositae of
Bahia, Brazil I. – Kew Bull. 48: 245-277.
Hind DJN. 1993b. A checklist of the Brazilian
Senecioneae (Compositae). – Kew Bull. 48: 279-295.
Hind DJN. 1994. New Compositae from the Serra
do Grão Mogol (Mun. Grão Mogol, Minas Gerais, Brazil) and the surrounding
area. – Kew Bull. 49: 511-522.
Hind DJN. 1999a. A new genus,
Semiria (Compositae: Eupatorieae), and a discussion of its affinities
within the subtribe Gyptidinae of Bahia, Brazil. – Kew Bull. 54: 425-435.
Hind DJN. 1999b. The tribe Senecioneae
(Compositae) in Bahia, Brazil, with descriptions of a new section and species
in Senecio. – Kew Bull. 54: 897-904.
Hind DJN. 1999c. A new species of
Lasiolaena (Compositae: Eupatorieae: Gyptidinae) and a synopsis of the
genus. – Kew Bull. 54: 915-925.
Hind DJN. 1999d. Notes on Chaptalia
(Compositae: Mutisieae) in Brazil. – Kew Bull. 54: 933-939.
Hind DJN. 2000a. A new species and a
commentary on the genus Trixis (Compositae: Mutisieae) in Bahia,
Brazil. – Kew Bull. 55: 381-386.
Hind DJN. 2000b. A new genus,
Catolesia (Compositae: Eupatorieae), and a discussion of its
affinities within the subtribe Gyptidinae of Bahia, Brazil. – Kew Bull. 55:
941-948.
Hind DJN. 2001. A new combination in
Amblysperma (Compositae: Mutisieae). – Kew Bull. 56: 711-713.
Hind DJN. 2004. Novaguinea
(Compositae: Astereae: Lagenophorinae), a new endemic genus to Papua,
Indonesia. Contributions to the flora of Mount Jaya XIII. – Kew Bull. 59:
177-188.
Hind DJN. 2009. Agrianthus carvalhoi
(Compositae: Eupatorieae: Gyptidinae), a new species from Bahia State, Brazil.
– Kew Bull. 64: 291-294.
Hind DJN. 2014a. A synopsis of
Calostephane (Compositae: Inuleae). – Kew Bull. 69: 9516.
Hind DJN. 2014b. A synopsis of
Amblysperma (Compositae: Mutisieae: Gerberinae), with some further
observations. – Kew Bull. 69: 9507.
Hind DJN. 2014c. A synopsis of the African
genus Lipotriche (Compositae: Heliantheae: Ecliptinae). – Kew Bull.
69: 9528.
Hind DJN, Boulos L. 2002. Four new
combinations in Pulicaria (Compositae: Inuleae). – Kew Bull. 57:
495-498.
Hind DJN, Jeffrey C. 1988.
Brachycome Cass. corr. Cass. and Lagenophora Cass. corr.
Cass. are correct. – Kew Bull. 43: 329-331.
Hind DJN, Johns RJ. 2003. Contributions to
the flora of Mount Jaya XI. New species of Papuacalia (Compositae:
Senecioneae). – Kew Bull. 58: 389-402.
Hind DJN, Jeffrey C, Pope GV (eds). 1995.
Advances in Compositae systematics. – Royal Botanic Gardens, Kew.
Hindmarsh MM, Blaxell DF. 1978. A new species
of Stylidium (Stylidiaceae) from the Sydney
region. – Telopea 1: 365-370.
Hobbs CR, Baldwin BG. 2013. Asian origin and
upslope migration of Hawaiian Artemisia (Compositae-Anthemideae). –
J. Biogeogr. 40: 442-454.
Höck F. 1894. Calyceraceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien IV(5), W. Engelmann, Leipzig,
pp. 84-86.
Hofer O, Greger H. 1984. Naturally occurring
sequiterpene-coumarin ethers VI. New sesquiterpene-isofraxidin ethers from
Achillea depressa. – Monatshefte Chemie 115: 477-483.
Hoffmann O. 1894. Compositae. – In: Engler
A, Prantl K (eds), Die natürlichen Pflanzenfamilien IV(5), W. Engelmann,
Leipzig, pp. 87-391; Hoffmann O. 1897. Nachträge zu IV(5), pp. 320-330.
Hoffmann O. 1896. Compostas da Africa
Portugueza. – Bol. Soc. Brot. 13: 11-35.
Holland A, Funk VA. 2006. A revision of
Cymbonotus (Compositae: Arctotideae, Arctotidinae). – Telopea 11:
266-275.
Holmgren I. 1919. Zytologische Studien über
die Fortpflanzung bei den Gattungen Erigeron und Eupatorium.
– Kungl. Sv. Vet. Akad. Handl. 59.
Holmes WC. 1982. Revision of the Old World
Mikania (Compositae). – Bot. Jahrb. Syst. 103: 211-246.
Holmes WC. 1991. Dioecy in Mikania
(Compositae: Eupatorieae). – Plant Syst. Evol. 175: 87-92.
Holmes WC, Pruski JF. 2000. New species of
Mikania (Compositae: Eupatorieae) from Ecuador and Peru. – Syst.
Bot. 25: 571-576.
Holub M, Poplawski J, Sedmera P, herout V.
1977. N-ethoxy-carbonyl-L-prolinamide, a new alkaloid from the leaves of
Arnica montana L. – Coll. Czech. Chem. Commun. 42: 151-154.
Holub M, Toman J, Herout V. 1987. The
phylogenetic relationships of the Asteraceae and Apiaceae based on phytochemical
characters. – Biochem. Syst. Ecol. 15: 321-326.
Holzapfel S. 1993. A revision of the genus
Picris (Asteraceae,
Lactuceae) s.l. in Australia. – Willdenowia 24: 97-218.
Holzapfel S, Lack HW. 1993. New species of
Picris (Asteraceae,
Lactuceae) from Australia. – Willdenowia 23: 181-191.
Hong L, Trusty J, Oviedo R, Anderberg AA,
Francisco-Ortego J. 2004. Molecular phylogenetics of the Caribbean genera
Rhodogeron and Sachsia (Asteraceae). – Intern. J. Plant
Sci. 165: 209-217.
Hong D-Y. 1995. The geography of the Campanulaceae: on the distribution
centres. – Acta Phytotaxon. Sin. 33: 521-536.
Hong D-Y. 2002. Two new species of Campanulaceae from South Africa.
– Taxon 51: 731-735.
Hong D-Y. 2010. Taxonomic notes on Chinese Campanulaceae. – Novon 20:
420-425.
Hong D-Y. 2015. A monograph of
Codonopsis and allied genera (Campanulaceae). – Science Press,
Beijing.
Hong D-Y, Ma L-M. 1991. Systematics of the
genus Cyananthus Wall. ex Royle. – Acta Phytotaxon. Sin. 29:
25-51.
Hong D-Y, Pan K-Y. 1998. The restoration of
the genus Cyclocodon (Campanulaceae) and its evidence
from pollen and seed-coat. – Acta Phytotaxon. Sin. 36: 106-110.
Hong D-Y, Wang Q. 2015. A new taxonomic
system of the Campanulaceae
s.s. – J. Syst. Evol. 53: 203-209.
Horn GS van. 1973. The taxonomic status of
Pentachaeta and Chaetopappa with a revision of
Pentachaeta. – Univ. Calif. Publ. Bot. 65: 1-41.
Horner HT Jr, Pearson C. 1978. Pollen wall
and aperture development in Helianthus annuus (Compositae,
Heliantheae). – Amer. J. Bot. 65: 293-309.
Hou-Liu SY. 1963. The chromosome counts of
some Taraxacum species. – Acta Bot. Neerl. 12: 76-81.
Howarth DG, Baum DA. 2002. Phylogenetic
utility of a nuclear intron from nitrate reductase for the study of closely
related plant species. – Mol. Phylogen. Evol. 23: 525-528.
Howarth DG, Baum DA. 2005. Genealogical
evidence of homoploid hybrid speciation in an adaptive radiation of
Scaevola (Goodeniaceae)
in the Hawaiian Islands. – Evolution 59: 948-961.
Howarth DG, Gustafsson MHG, Baum DA, Motley
TJ. 2003. Phylogenetics of the genus Scaevola (Goodeniaceae): implications for
dispersal patterns across the Pacific Basin and colonization of the Hawaiian
Islands. – Amer. J. Bot. 90: 915-923.
Howell JT. 1929. A systematic study of the
genus Lessingia Cham. – Univ. Calif. Publ. Bot. 16: 1-44.
Howell JT. 1941. The genus Scalesia.
– Proc. Calif. Acad. Sci., Ser. 4, 22: 221-271.
Howis S, Barker NP, Mucina L. 2009. Globally
grown, but poorly known: species limits and biogeography of Gazania
Gaertn. (Asteraceae) inferred from
chloroplast and nuclear DNA sequence data. – Taxon 58: 871-882.
Huang Y, An Y-M, Meng S-Y, Guo Y-P, Rao G-Y.
2017. Taxonomic status and phylogenetic position of Phaeostigma in the
subtribe Artemisiinae (Asteraceae). – J. Syst. Evol. 55:
426-436.
Huber W, Leuchtmann A. 1992. Genetic
differentiation of the Erigeron species (Asteraceae) in the Alps: a case of
unusual allozymic uniformity. – Plant Syst. Evol. 183: 1-16.
Huber W, Zhang H. 1991. Morphologische und
chemotaxonomische Untersuchungen an den Erigeron-Arten der Alpen. –
Ber. Geobot. Inst. ETH, Stiftung Rübel, Zürich 57: 116-164.
Huber-Morath A. 1972. Die anatolischen Arten
der Gattung Cousinia Cass. – Ber. Schweiz. Bot. Ges. 82: 223-268.
Humbert H. 1923. Les Composées de
Madagascar. – Mém. Soc. Linn. Normandie 25: 1-336.
Humbert H. 1962. 189ème Famille
– Composées 2. – In: Humbert H (ed), Flore de Madagascar et des Comores,
Firmin-Didot, Paris, pp. 339-622.
Humphries CJ. 1973. A taxonomic study of the
genus Argyranthemum. – Ph.D. diss., University of Reading,
England.
Humphries CJ. 1975. Cytological studies in
the Macaronesian genus Argyranthemum (Compositae: Anthemideae). –
Bot. Not. 128: 239-255.
Humphries CJ. 1976a. A revision of the
Macaronesian genus Argyranthemum Webb ex Schultz-Bip.
(Compositae-Anthemideae). – Bull. Brit. Mus. (Nat. Hist.), Bot. 5:
147-240.
Humphries CJ. 1976b. Evolution and endemism
in Argyranthemum Webb ex Schultz Bip. (Compositae-Anthemideae). –
Bot. Macaronésica 1: 25-50.
Humphries CJ. 1977. A new genus of the
Compositae from North Africa. – Bot. Not. 130: 155-161.
Humphries CJ. 1979. A revision of the genus
Anacyclus L. (Compositae: Anthemideae). – Bull Brit. Mus. (Nat.
Hist.), Bot. 7: 83-142.
Humphries CJ. 1981. Cytogenetic and cladistic
studies in Anacyclus (Compositae: Anthemideae). – Nord. J. Bot. 1:
83-96.
Humphries CJ, Murray BG, Bocquet G, Vasudevan
KN. 1978. Chromosome numbers of phanerogams from Morocco and Algeria. – Bot.
Not. 131: 391-406.
Hunger S. 1996. The Pluchea
tetranthera complex (Compositae, Plucheeae) from Australia. –
Willdenowia 26: 273-282.
Hunger S. 1997. A survey of the genus
Pluchea (Compositae, Plucheeae) in Australia. – Willdenowia 27:
207-223.
Hunziker JH, Wulff A, Xifreda CC, Escobar A.
1989. Estudios cariológicos en Compositae V. – Darwiniana 29: 25-39.
Hunziker JH, Escobar A, Xifred CC, Gamerro
JC. 1990. Estudios cariológicos en Compositae VI. – Darwiniana 30:
115-121.
Hürlimann H. 1964. Phelline. –
In: Guillaumin A (ed), Résultats scientifiques de la mission franco-suisse de
botanique en Nouvelle-Calédonie (1950-1952) III, Mém. Mus. Natl. Hist. Nat.,
B, Bot. 15: 63-65.
Hutchins G. 1994. The genus Corokia.
– The Plantsman 15: 225-235.
Hutchinson J. 1916a. Notes on African
Compositae II. Brachymeris, DC., and Marasmodes, DC. – Kew
Bull. 1916: 171-175.
Hutchinson J. 1916b. Notes on African
Compositae III. Pentzia, Thunb. – Kew Bull. 1916: 241-254.
Hutchinson J. 1917. Notes on African
Compositae IV. Matricaria, Linn., and Chrysanthemum, DC. –
Kew Bull. 1917: 111-118.
Hutchinson J, Phillips EP. 1917. A revision
of the genus Pteronia (Compositae). – Ann. South Afr. Mus. 9:
277-329.
Huziwara Y. 1957. Karyotype analysis in some
genera of Compositae III. The karyotype of the Aster ageratoides
group. – Amer. J. Bot. 44: 783-790.
Huziwara Y. 1959. Chromosomal evolution in
the subtribe Asterinae. – Evolution 13: 188-193.
Huziwara Y. 1962a. Karyotype analysis in some
genera of Compositae VIII. Further studies on the chromosomes of
Aster. – Amer. J. Bot. 49: 116-119.
Huziwara Y. 1962b. Karyotype analysis in some
genera of Compositae IX. Chromosomes of European species of Aster. –
Bot. Mag. (Tokyo) 75: 143-149.
Huziwara Y. 1965. Chromosome analysis in the
tribe Astereae. – Jap. J. Genet. 40: 63-71.
Huziwara Y. 1967. Chromosomal evolution in
Aster and its related genera. – Taxon 16: 301-303.
Iljin M. 1960. Species novae generis
Jurinea Cass. ex Asia Media. – Bot. Mater. Gerb. Bot. Inst. Komarova
Akad. Nauk S.S.S.R. 20: 344-355. [In Russian]
Inceer H, Hayirlioglu-Ayaz S. 2007.
Chromosome numbers in the tribe Anthemideae (Asteraceae) from North-East Anatolia.
– Bot. J. Linn. Soc. 153: 203-211.
Inceer H, Hayirlioglu-Ayaz S. 2009.
Tripleurospermum ziganaense (Asteraceae, Anthemideae), a new
species from North-East Anatolia, Turkey. – Bot. J. Linn. Soc. 158:
696-700.
Inoue K. 1988. Pattern of breedning-system
change in the Izu Islands in Campanula punctata: bumblebee-absence
hypothesis. – Plant Spec. Biol. 3: 125-128.
Inoue K, Maki M, Masuda M. 1995. Evolution of
Campanula flowers in relation to insect pollination on islands. –
In: Lloyd DG, Barrett SCH (eds), Floral biology: studies on floral evolution in
animal-pollinated plants, Chapman and Hall, New York, pp. 377-400.
Inoue N, Tobe H. 1999. Integumentary studies
in Menyanthaceae (Campanulales sensu lato). – Acta
Phytotaxon. Geobot. 50: 75-79.
Isawumi MA. 2008. The status of generic
revision in the African Vernonieae (Asteraceae). – Compositae
Newsletter 46: 27-48.
Isawumi MA, El-Ghazaly G, Nordenstam B. 1996.
Polen morphology, floral microcharacters and taxonomy of the genus
Baccharoides Moench (Vernonieae: Asteraceae). – Grana 35:
205-230.
Ito K, Sakakibara Y, Haruna M. 1982. Seven
guaianolides from Eupatorium chinense. – Phytochemistry 21:
715-720.
Ito M, Watanabe K, Kita Y, Kawahara T,
Crawford DJ, Yahara T. 2000. Phylogeny and phytogeography of
Eupatorium (Eupatorieae, Asteraceae): insights from sequence
data of the nrDNA ITS regions and cpDNA RFLP. – J. Plant Res. 113: 79-89.
Ito M, Yahara T, King RM, Watanabe K, Oshita
S, Yokoyama J, Crawford DJ. 2000. Molecular phylogeny of Eupatorieae (Asteraceae) estimated from cpDNA RFLP
and its implication for the polyploid origin hypothesis of the tribe. – J.
Plant Res. 113: 91-96.
Izuzquiza A, Nieto Feliner G. 1991.
Cytotaxonomic notes on the genus Leontodon (Asteraceae, Hypochoeridinae). –
Willdenowia 21: 215-224.
Jabaily RS, Shepherd KA, Gardner AG,
Gustafsson MHG, Howarth DG, Motley TJ. 2014. Historical biogeography of the
predominantly Australian plant family Goodeniaceae. – J. Biogeogr. 41:
2057-2067.
Jabbour F, Damerval C, Nadot S. 2008.
Evolutionary trends in the flowers of Asteridae: is polyandry an alternative to
zygomorphy? – Ann. Bot. 102: 153-165.
Jackson JD. 1975. A revision of the genus
Archibaccharis Heering (Compositae-Astereae). – Phytologia 32:
81-105.
Jackson RC. 1960. A revision of the genus
Iva L. – Univ. Kansas Sci. Bull. 41: 793-876.
Jackson RC. 1967. Biosystematic studies in
Haplopappus (Compositae). – Taxon 16: 303-304.
Jackson RC, Dimas CT. 1981. Experimental
evidence for systematic placement of the Haplopappus phyllocephalus
complex (Compositae). – Syst. Bot. 6: 8-14.
Jackson RC, Skvarla JJ, Chissoe WF. 2000. A
unique pollen wall mutation in the family Compositae: ultrastructure and
genetics. – Amer. J. Bot. 87: 1571-1577.
Jackson WD, Wiltshire RJE. 2001. Historical
taxonomy and a resolution of the Stylidium graminifolium complex (Stylidiaceae) in Tasmania. –
Aust. Syst. Bot. 14: 937-969.
Jacono C. 2002. Florida’s floating-hearts:
know Nymphoides. – Aquaphyte 22: 9.
Jafari F, Osaloo SK, Mozffarian V. 2015.
Molecular phylogeny of the tribe Astereae (Asteraceae) in SW Asia based on nrDNA
ITS and cpDNA psbA-trnH sequences. – Willdenowia 45:
77-92.
Jakubowsky G, Mucina L. 2007. Phylogeny of
the South African centred plant genus Cotula (Asteraceae). – South Afr. J. Bot.
73: 292.
Jakupovic J, Paredes L, Bohlmann F, Watson
LM. 1988. Prenyl flavanes from Marshallia species. – Phytochemistry
27: 3273-3275.
Jakupovic J, Zdero C, Boeker R, Warning U,
Bohlmann F, Jones SB. 1987. Vernocistifolide und andere Sesquiterpenlactone aus
Vernonia und verwandten Arten. – Liebigs Ann. Chemie 1987:
111-123.
James SH. 1979. Chromosome numbers and
genetic systems in the trigger plants of Western Australia (Stylidium;
Stylidiaceae). – Aust. J. Bot.
27: 17-25.
Janka V von. 182. Odontolophus, eine
ausgezeichnete Gattung. – Österr. Bot. Zeitschr. 32: 280-281.
Jansen RK. 1981. Systematics of
Spilanthes (Compositae: Heliantheae). – Syst. Bot. 6: 231-257.
Jansen RK. 1985a. The systematics of
Acmella (Asteraceae-Heliantheae). – Syst.
Bot. Monogr. 8.
Jansen RK. 1985b. Systematic significance of
chromosome numbers in Acmella (Asteraceae). – Amer. J. Bot. 72:
1835-1841.
Jansen RK. 1991. Phylogeny and character
evolution in the Asteraceae based
on chloroplast DNA restriction site mapping. – Syst. Bot. 16: 98-115.
Jansen RK, Kim K-J. 1996. Implications of
chloroplast DNA data for the classification and phylogeny of the Asteraceae. – In: Hind DJN, Beentje
HJ (eds), Compositae: systematics. Proceedings of the International Compositae
Conference, Kew, 1994, vol. 1, Royal Botanic Gardens, Kew, pp. 317-339.
Jansen RK, Palmer JD. 1987a. Chloroplast DNA
from lettuce and Barnadesia (Asteraceae): structure, gene
localization, and characterization of a large inversion. – Curr. Genet. 11:
553-564.
Jansen RK, Palmer JD. 1987b. A chloroplast
DNA inversion marks an ancient evolutionary split in the sunflower family (Asteraceae). – Proc. Natl. Acad.
Sci. U.S.A. 84: 5818-5822.
Jansen RK, Palmer JD. 1988. Phylogenetic
implications of chloroplast DNA restriction site variation in the Mutisieae (Asteraceae). – Amer. J. Bot. 75:
753-766.
Jansen RK, Stuessy TF. 1980. Chromosome
counts of Compositae from Latin America. – Amer. J. Bot. 67: 585-594.
Jansen RK, Harriman NA, Urbatsch LE. 1982.
Squamopappus gen. nov. and redefinition of Podachaenium
(Compositae: Heliantheae). – Syst. Bot. 7: 476-483.
Jansen RK, Stuessy TF, Diaz-Piedrahita S,
Funk VA. 1984. Recuentos cromosómicos en Compositae de Colombia. – Caldasia
14: 7-20.
Jansen RK, Smith EB, Crawford DJ. 1987. A
cladistic study of North American Coreopsis (Asteraceae: Heliantheae). – Plant
Syst. Evol. 157: 73-84.
Jansen RK, Palmer JD, Michaels HJ. 1988.
Investigations of chloroplast DNA variation in the Asteraceae. – Compositae Newsl. 15:
2-9.
Jansen RK, Holsinger KE, Michaels HJ, Palmer
JD. 1990. Phylogenetic analysis of chloroplast DNA restriction site data at
higher taxonomic levels: an example from the Asteraceae. – Evolution 44:
2089-2105.
Jansen RK, Michaels HJ, Palmer JD. 1991.
Phylogeny and character evolution in the Asteraceae based on chloroplast DNA
restriction site mapping. – Syst. Bot. 16: 98-115.
Jansen RK, Wallace RS, Kim K-J, Chambers KL.
1991. Systematic implications of chloroplast DNA variation in the subtribe
Microseridinae (Asteraceae:
Lactuceae). – Amer. J. Bot. 78: 1015-1027.
Jansen RK, Michaels HJ, Wallace RS, Kim K-J,
Keeley SC, Watson LE, Palmer JD. 1992. Chloroplast DNA variation in the Asteraceae: phylogenetic and
evolutionary implications. – In: Soltis PS, Soltis DE, Doyle JJ (eds),
Molecular systematics of plants, Chapman Hall, New York, pp. 252-279.
Jara-Arancio P, Vidal PM, Panero JL,
Marticorena A, Arancio G, Arroyo MTK. 2017. Phylogenetic reconstruction of the
South American genus Leucheria Lag. (Asteraceae, Nassauvieae) based on
nuclear and chloroplast DNA sequences. – Plant Syst. Evol. 303: 221-232.
Jara-Arancio P, Vidal PM, Arroyo MTK. 2018.
Phylogenetic reconstruction of the genus Triptilion (Asteraceae, Nassauvieae) based on
nuclear and chloroplast DNA sequences. – J. Syst. Evol. 56: 120-128.
Jay M. 1969. Contribution biochimique à la
connaissance taxonomique et phylogénetique des Saxifragacées et familles
affinés. – Ph.D. diss., l’Université de Lyon, France.
Jeanmonod D. 2003. Le groupe du Senecio
leucanthemifolius en Corse, avec description d’une nouvelle espèce:
S. serpentinicola Jeanm. – Candollea 58: 429-459.
Jeffrey C. 1966. Notes in Compositae I. The
Cichorieae in East Tropical Africa. – Kew Bull. 18: 427-486.
Jeffrey C. 1967. Notes on Compositae II. The
Mutisieae in East Tropical Africa. – Kew Bull. 21: 177-223.
Jeffrey C. 1977. Corolla forms in the
Compositae: some evolutionary and taxonomic speculations. – In: Heywood VH,
Harborne JB, Turner BL (eds), The biology and chemistry of the Compositae,
Academic Press, London, pp. 111-118.
Jeffrey C. 1979. Generic and sectional limits
in Senecio (Compositae) II. Evaluation of some recent studies. – Kew
Bull. 34: 49-58.
Jeffrey C. 1982. Generic names of Compositae
IV. – Compositae Newsl. 13: 28-38.
Jeffrey C. 1986. Notes on Compositae IV. The
Senecioneae in East Tropical Africa. – Kew Bull. 41: 873-943.
Jeffrey C. 1988. Notes on Compositae V. The
Vernonieae in East Tropical Africa. – Kew Bull. 43: 195-277.
Jeffrey C. 1990. Generic names of Compositae
VII. D. – Compositae Newslett. 18: 2-28.
Jeffrey C. 1992a. Notes on Compositae VI. The
tribe Senecioneae (Compositae) in the Mascarene Islands with an annotated world
check-list of the genera of the tribe. – Kew Bull. 47: 49-109.
Jeffrey C. 1992b. The tribe Senecioneae
(Compositae): corrections. – Kew Bull. 47: 292.
Jeffrey C. 1995. Compositae systematics
1975-1993, developments and desiderata. – In: Hind DJN, Jeffrey C, Pope GV
(eds), Advances in Compositae systematics, Royal Botanic Gardens, Kew, pp.
3-21.
Jeffrey C. 1997. What is Emilia
coccinea (Sims) G. Don (Compositae)? A revision of the large-headed
Emilia species of Africa. – Kew Bull. 52: 205-212.
Jeffrey C. 2002. Systematics of Compositae at
the beginning of the 21st Century. – Bot. Žurn. 87: 1-15. [In
Russian]
Jeffrey C. 2004. Systema compositarum
(asteracearum) nova. – Bot. Žurn. 89: 1817-1822.
Jeffrey C. 2009. Evolution of Compositae
flowers. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics,
evolution, and biogeography of Compositae, International Association for Plant
Taxonomists, Wien, pp. 131-138.
Jeffrey C, Chen Y-L. 1984. Taxonomic studies
on the tribe Senecioneae (Compositae) of eastern Asia. – Kew Bull. 39:
205-446.
Jeffrey C, Halliday P, Wilmot-Dear M, Jones
SW. 1978. Generic and sectional limits in Senecio (Compositae) I.
Progress report. – Kew Bull. 32: 47-67.
Jeppesen S. 1981. 187. Campanulaceae, 188. Lobeliaceae,
189B. Goodeniaceae. – In:
Harling G, Sparre B (eds), Flora of Ecuador 14, Swedish Natural Science
Research Council, Stockholm, pp. 1-183.
Jimenez Rodríguez F, Katinas L, Telleria MC,
Crisci JV. 2004. Salcedoa gen. nov., a biogeographic enigma in the
Caribbean Mutisieae (Asteraceae).
– Syst. Bot. 29: 987-1002.
John D. 1996. Control of fructose metabolism
in the Compositae. – In: Caligari P, Hind DJN (eds), Compositae: Biology and
utilization. Proceedings of the International Compositae Conference, Kew, 1994,
vol. 2, Royal Botanic Gardens, Kew, pp. 111-119.
Johnson DE. 1978. Systematics of
Eriophyllinae (Compositae). – Ph.D. diss., University of California,
Berkeley, California.
Johnson MF. 1971. A monograph of the genus
Ageratum L. (Compositae-Eupatorieae). – Ann. Missouri Bot. Gard. 58:
6-88.
Jones AG. 1976. Observations on the shape and
exposure of style branches in the Astereae (Compositae). – Amer. J. Bot. 63:
259-262.
Jones AG. 1977. New data on chromosome
numbers in Aster Section Heterophylli (Asteraceae) and their phylogenetic
implications. – Syst. Bot. 2: 334-347.
Jones AG. 1978. The taxonomy of
Aster section Multiflori (Asteraceae) II. Biosystematic
investigations. – Rhodora 80: 453-490.
Jones AG. 1980a. A classification of the New
World species of Aster (Asteraceae). – Brittonia 32:
230-239.
Jones AG. 1980b. Data on chromosome numbers
in Aster (Asteraceae),
with comments on the status and relationships of certain North American
species. – Brittonia 32: 240-261.
Jones AG. 1982. Virgulus Raf.
vis-à-vis Aster L. (Asteraceae). – Taxon 31:
714-715.
Jones AG. 1985. Chromosomal features as
generic criteria in the Astereae. – Taxon 34: 44-54.
Jones AG, Smogor A. 1983. Chromosome counts
of and notes on some Old World asters (Asteraceae). – Phytologia 53:
429-431.
Jones AG, Young DA. 1983. Generic concepts of
Aster (Asteraceae): a
comparison of cladistic, phenetic, and cytological approaches. – Syst. Bot.
8: 71-84.
Jones KE, Korotkova N, Petersen J, Henning T,
Borsch T, Kilian N. 2017. Dynamic diversification history with rate upshifts in
Holarctic bell-flowers (Campanula and allies). – Cladistics 33:
637-666.
Jones RL. 1983. A systematic study of
Aster section Patentes (Asteraceae). – Sida 10: 41-81.
Jones Jr SB. 1970. Scanning electron
microscopy of pollen as an aid to the systematics of Vernonia. –
Bull. Torrey Bot. Club 97: 325-335.
Jones Jr SB. 1973. Revision of
Vernonia Section Eremosis (Compositae) in North America. –
Brittonia 25: 86-115.
Jones Jr SB. 1974. Vernonieae (Compositae)
chromosome numbers. – Bull. Torrey Bot. Club 101: 31-34.
Jones Jr SB. 1976. Cytogenetics and
affinities of Vernonia (Compositae) from the Mexican highlands and
eastern North America. – Evolution 30: 455-462.
Jones Jr SB. 1977. Vernonieae – systematic
review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae 2, Academic Press, London, pp. 503-521.
Jones Jr SB. 1979a. Synopsis and pollen
morphology of Vernonia in the New World. – Rhodora 81: 425-447.
Jones Jr SB. 1979b. Chromosome numbers of
Vernonieae (Compositae). – Bull. Torrey Bot. Club 106: 79-84.
Jones Jr SB. 1981. Synoptic classification
and pollen morphology of Vernonia (Compositae) in the Old World. –
Rhodora 83: 59-75.
Juel HO. 1900. Vergleichende Untersuchungen
über typische und parthenogenetische Fortpflanzung bei der Gattung
Antennaria. – Kungl. Svenska Vet. Akad. Handl. 33(5): 1-59.
Kadereit JW. 1983. Experimental study and
revision of Senecio Section Obaejacae DC. – Ph.D. diss.,
Cambridge University, England.
Kadereit JW. 1989. Chloroplast DNA,
cladistics and the phylogeny of the Asteraceae. – Bot. Acta 102:
7-10.
Kadereit JW. 2006 [2007]a. Asterales:
introduction and conspectus. – In: Kubitzki K, Kadereit JW, Jeffrey C (eds),
The families and genera of vascular plants VIII. Flowering plants. Eudicots.
Asterales, Springer, Berlin, Heidelberg, New York, pp. 1-6.
Kadereit G. 2006 [2007]b. Menyanthaceae. – In: Kubitzki K,
Kadereit JW, Jeffrey C (eds), The families and genera of vascular plants VIII.
Flowering plants. Eudicots. Asterales, Springer, Berlin, Heidelberg, New York,
pp. 599-604.
Kadereit JW, Jeffrey C. 1996. A preliminary
analysis of cpDNA variation in the tribe Senecioneae (Compositae). – In: Hind
DJN, Beentje HJ (eds), Compositae: systematics. Proceedings of the
International Compositae Conference, Kew, 1994, I, Royal Botanic Gardens, Kew,
pp. 349-360.
Kadereit JW, Uribe-Convers S, Westberg E,
Comes HP. 2006. Reciprocal hybridization at different times between Senecio
flavus and Senecio glaucus gave rise to two polyploid species in
north Africa and south-west Asia. – New Phytol. 169: 431-441.
Källersjö M 1985. Fruit structure and
generic delimitation of Athanasia (Asteraceae-Anthemideae) and related
South African genera. – Nord. J. Bot. 5: 527-542.
Källersjö M. 1988. A generic
re-classification of Pentzia Thunb. (Compositae-Anthemideae) from
southern Africa. – Bot. J. Linn. Soc. 96: 299-322.
Kalpoutzakis E, Constantinidis T. 2004. A new
species of Centaurea (sect. Phalolepis, Compositae: Cardueae)
from eastern Peloponnisos, Greece. – Bot. J. Linn. Soc. 146:375-383.
Kamelin RV. 1993. Rod Lipschitziella
R. Kam. gen. nov. – In: Vvedenskij AI (ed), Conspectus florae Asiae mediae
10, Taškent, pp. 371, 632. [In Russian]
Kamelin RV. 1999. A Middle-Asiatic species of
the section Frolovia (DC.) Kitam. of the genus Saussurea DC.
– Turczaninowia 2: 25-32. [In Russian]
Kamelin RV, Kovalevskaja SS. 1993. Rod
Frolovia (DC.) Lipsch. – In: Vvedenskij AI (ed), Conspectus florae
Asiae mediae 10, Taškent, pp. 353-354.
Kandemir A. 2007. A new Campanula
(Campanulaceae) from east
Anatolia, Turkey. – Nord. J. Bot. 25: 53-57.
Kaneda N, Kohda H, Yamasaki K, Tanaka O,
Nishi K. 1978. Paniculosides I-V, diterpene glycosides from Stevia
ovata. – Chem. Pharm. Bull. 26: 2266-2267.
Kapil RN, Bhatnagar AK. 1974. Stomata on
leaves and floral parts of Corokia. – Bot. Jahrb. Syst. 94:
257-266.
Kapil RN; Bhatnagar AK. 1992. Embryology and
systematic position of Corokia Cunn. – In: Batygina TB (ed),
Proceedings of the XI International symposium on embryology and seed
reproduction, Leningrad, 1990, Nauka, St. Petersburg, pp. 246-247.
Kapil RN, Vijayaraghavan MR. 1965. Embryology
of Pentaphragma horsfieldii (Miq.) Airy Shaw with a discussion on the
systematic position of the genus. – Phytomorphology 15: 93-102.
Kaplan DR. 1967. Floral morphology,
organogenesis, and interpretation of the inferior ovary in Downingia
bacigalupii. – Amer. J. Bot. 54: 1274-1290.
Kaplan DR. 1968. Structure and development of
the perianth in Downingia bacigalupii. – Amer. J. Bot. 55:
406-420.
Kapoor BM, Beaudry JR. 1966. Studies on
Solidago VII. The taxonomic status of the taxa Brachychaeta,
Brintonia, Chrysoma, Euthamia, Oligoneuron
and Petradoria in relation to Solidago. – Can. J. Genet.
Cytol. 8: 422-443.
Karaman-Castro V, Urbatsch LE. 2009.
Phylogeny of Hinterhubera group and related genera (Hinterhuberinae:
Astereae) based on the nrDNA ITS and ETS sequences. – Syst. Bot. 34:
805-817.
Karanović D, Luković J, Zorić L, Anačkov
G, Boža P. 2015. Taxonomic status of Aster, Galatella and
Tripolium (Asteraceae) in
view of anatomical and micro-morphological evidence. – Nord. J. Bot. 33:
484-497.
Kårehed J. 2006a [2007]. Alseuosmiaceae. – In: Kubitzki
K, Kadereit JW, Jeffrey C (eds), The families and genera of vascular plants
VIII. Flowering plants. Eudicots. Asterales, Springer, Berlin, Heidelberg, New
York, pp. 7-12.
Kårehed J. 2006b [2007]. Argophyllaceae. – In: Kubitzki
K, Kadereit JW, Jeffrey C (eds), The families and genera of vascular plants
VIII. Flowering plants. Eudicots. Asterales, Springer, Berlin, Heidelberg, New
York, pp. 13-18.
Kårehed J, Lundberg J, Bremer B, Bremer K.
2000. Evolution of the Australasian families Alseuosmiaceae, Argophyllaceae, and Phellinaceae. – Syst. Bot. 24:
660-682.
Karis PO. 1989. Systematics of the genus
Metalasia (Asteraceae-Gnaphalieae). – Opera
Bot. 99: 1-150.
Karis PO. 1990. Three new genera of the Asteraceae-Gnaphalieae from the Cape
Region. – Bot. J. Linn. Soc. 102: 23-36.
Karis PO. 1992. Hoplophyllum DC.,
the sister group to Eremothamnus O. Hoffm. (Asteraceae)? – Taxon 41:
193-198.
Karis PO. 1993a. Morphological phylogenetics
of the Asteraceae-Asteroideae,
with notes on character evolution. – Plant Syst. Evol. 186: 69-93.
Karis PO. 1993b. The Heliantheae sensu lato
(Asteraceae), clades and
classification. – Plant Syst. Evol. 188: 139-195.
Karis PO. 1995. Cladistics of the subtribe
Ambrosiinae (Asteraceae:
Heliantheae). – Syst. Bot. 20: 40-54.
Karis PO. 1996. Phylogeny of the Asteraceae-Asteroideae revisited. –
In: Hind DJN, Beentje HJ (eds), Compositae: systematics. Proceedings of the
International Compositae Conference, Kew, 1994, vol. 1, Royal Botanic Gardens,
Kew, pp. 41-47.
Karis PO. 1998. Apostates Lander (Asteraceae-Astereae), transferred to
the Heliantheae sensu lato. – Bot. Jahrb. Syst. 120: 131-135.
Karis PO. 2001. New evidence for the
systematic position of Gundelia L. with notes on delimitation of
Arctot[id]eae (Asteraceae). –
Taxon 50: 105-114.
Karis PO. 2006. Morphological data indicates
two major clades of the subtribe Gorteriinae (Asteraceae-Arctotideae). –
Cladistics 22: 199-221.
Karis PO, Källersjö M, Bremer K. 1992.
Phylogenetic analysis of the Cichorioideae (Asteraceae), with emphasis on the
Mutisieae. – Ann. Missouri Bot. Gard. 79: 416-427.
Karis PO, Anderberg AA, Nordenstam B. 1993.
Resurrection and systematic position of Pentalepis F. Muell. (Asteraceae-Heliantheae). – Aust.
Syst. Bot. 6: 149-153.
Karis PO, Eldenäs P, Källersjö M. 2001.
New evidence for the systematic position of Gundelia L. with notes on
delimitation of Arctot[id]eae (Asteraceae). – Taxon 50:
105-114.
Karis PO, Funk VA, McKenzie RJ, Barker NP,
Chan R. 2009. Arctotideae. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ
(eds), Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 385-410.
Kasinathan P, Kumari SN. 2001. Systematic
position of Nymphoides hydrophylla. – In: Maheshwari JK, Jain AP
(eds), Recent researches in plant anatomy and morphology, Scientific Publ.,
Jodhpur, pp. 155-160.
Katinas L. 1998. The Mexican Chaptalia
hintonii is a Gerbera (Asteraceae, Mutisieae). – Novon 8:
380-385.
Katinas L. 2000. Implications of
morphological phylogenetics for the placement of the genera
Adenocaulon and Eriachaenium (Asteraceae). – Plant Syst. Evol.
223: 229-250.
Katinas L. 2004. Amblysperma should
be retained under Trichocline (Asteraceae, Mutisieae). – Taxon 53:
108-112.
Katinas L. 2008. The genus
Pachylaena (Asteraceae,
Mutisieae). – Bot. J. Linn. Soc. 157: 373-380.
Katinas L, Crisci JV. 2000. Cladistic and
biogeographic analyses of the genera Moscharia and
Polyachyrus (Asteraceae,
Mutisieae). – Syst. Bot. 25: 33-46.
Katinas L, Crisci JV, Jabaily RS, Williams C,
Walker J, Drew B, Bonifacino JM, Sytsma KJ. 2008. Evolution of secondary heads
in Nassauviinae (Asteraceae,
Mutisieae). – Amer. J. Bot. 95: 229-240.
Katinas L, Pruski J, Sancho G, Tellería MC.
2008. The subfamily Mutisioideae (Asteraceae). – Bot. Rev. 74:
469-716.
Katinas L, Sancho G, Tellería MC, Crisci JV.
2009. Mutisieae sensu stricto (Mutisioideae sensu stricto). – In: Funk VA,
Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and biogeography
of Compositae, International Association for Plant Taxonomists, Wien, pp.
229-248.
Katinas L, Sancho G, Vitali MS. 2013. A
revision of Lophopappus (Asteraceae, Nassauvieae). –
Phytotaxa 103: 25-45.
Katinas L, Hernández MP, Arambarri AM, Funk
VA. 2016. The origin of the bifurcating style in Asteraceae (Compositae). – Ann.
Bot. 117: 1009-1021.
Kausik SB. 1939. A cytological study of
Scaevola lobelia Linn. – Proc. Indian Acad. Sci., Sect. B, 9:
39-48.
Kausik SB, Subramanyam K. 1946. Development
of endosperm in Lobelia nicotianaefolia Heyne. – Curr. Sci. 3:
78-79.
Kawatani T, Ohno T. 1964. Chromosome numbers
in Artemisia. – Bull. Natl. Inst. Hygien. Sci. Tokyo 82: 183-193.
Kazmi SMA. 1963-1964. Revision der Gattung
Carduus (Compositae) I-II. – Mitt. Bot. Staatssamml. München 5:
139-198, 279-550.
Keck DD. 1936. The Hawaiian silverswords.
Systematics, affinities, and phytogeographic problems of the genus
Argyroxiphium. – Occas. Papers B. P. Bishop Mus. 11(19): 1-38.
Keck DD. 1958. Taxonomic notes on the
California flora. – Aliso 4: 102-103.
Keeley SC. 1982. Morphological variation and
species recognition in the Neotropical taxon Vernonia arborescens
(Compositae). – Syst. Bot. 7: 71-84.
Keeley SC, Chan K. 2003. The Vernonieae:
searching for a new paradigm. – Compositae Newslett. 40: 19.
Keeley SC, Jansen RK. 1991. Evidence from
chloroplast DNA for the recognition of a new tribe, Tarchonantheae, and the
tribal placement of Pluchea (Asteraceae). – Syst. Bot. 16:
173-181.
Keeley SC, Jansen RK. 1995. Chloroplast DNA
restriction site variation in the Vernonieae (Asteraceae), an initial appraisal of
the relationship of New and Old World taxa and the monophyly of
Vernonia. – Plant Syst. Evol. 193: 249-265.
Keeley SC, Jones Jr SB. 1977. Taxonomic
implications of external pollen morphology to Vernonia (Compositae) in
the West Indies. – Amer. J. Bot. 64: 576-584.
Keeley SC, Jones Jr SB. 1979. Distribution of
pollen types in Vernonia (Vernonieae: Compsitae). – Syst. Bot. 4:
195-202.
Keeley SC, Robinson H. 2009. Vernonieae. –
In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and
biogeography of Compositae, International Association for Plant Taxonomists,
Wien, pp. 439-469.
Keeley SC, Forsman ZH, Chan R. 2007. A
phylogeny of the “evil tribe” (Vernonieae: Compositae) reveals Old/New
World long distance dispersal: support from separate and combined datasets
(trnL-F, ndhF, ITS). – Mol. Phylogen. Evol. 44: 89-103.
Keil DH, Stuessy TF. 1981. Systematics of
Isocarpha (Compositae: Eupatorieae). – Syst. Bot. 6: 258-287.
Keil DJ, Luckow MA, Pinkava DJ. 1988.
Chromosome studies in Asteraceae
from the United States, Mexico, The West Indies, and South America. – Amer.
J. Bot. 75: 652-668.
Kelch DG, Baldwin BG. 2003. Phylogeny and
ecological radiation of New World thistles (Cirsium,
Cardueae-Compositae) based on ITS and ETS rDNA sequence data. – Mol. Ecol.
12: 141-151.
Kenneally KF, Lowrie A. 1994a. Rediscovery of
the presumed extinct triggerplant Stylidium merrallii (Stylidiaceae) with an amended
description of the species and its conservation status. – West. Aust. Natur.
19: 269-277.
Kenneally KF, Lowrie A. 1994b. Stylidium
costulatum (Stylidiaceae),
a new tropical species of triggerplant from the Kimberley, Western Australia,
and the lectotypification of S. floodii. – Nuytsia 9: 343-349.
Khanjian NS. 1991. On the position of the
genus Ursinia in the system of the family Asteraceae. – Bot. Žurn. 76:
1728-1733.
Khanjian NS. 1992. The taxonomic significance
of the achene’s structure in the subtribe Anthemideae (Asteraceae). – Bot. Žurn. 77:
89-98.
Khanminchun VM, Krasnoborov IM. 1984. Novyj
vid roda Saussurea DC. (Asteraceae) iz Altae-Sajanskoj
provincii. – Izv. Sibirsk. Otd. Akad. Nauk S.S.S.R. Ser. Biol. Nauk. 13:
14-16.
Kiang A-K, Sim K-Y, Yoong S-W. 1968.
Constituents of Mikania cordata II. – Phytochemistry 7:
1035-1037.
Kiers AM. 2000. Endive, chicory, and their
wild relatives. a systematic and phylogenetic study of Cichorium (Asteraceae). – Gorteria, Suppl.
5.
Kiers AM, Mes THM, Meijden R van der,
Bachmann K. 2000. Morphologically defined Cichorium (Asteraceae) species reflect lineages
based on chloroplast and nuclear (ITS) DNA data. – Syst. Bot. 24: 645-659.
Kiew R. 1990. Three new species of
Pentaphragma from Borneo. – Kew Bull. 45: 545-554.
Kilian N. 1988. Die Lactuceae (Compositae)
der Kapverdischen Inseln (W-Afrika). – Willdenowia 18: 113-216.
Kilian N. 1997. Revision of Launaea
Cass. (Compositae, Lactuceae, Sonchinae). – Englera 17: 1-478.
Kilian N. 1999a. Pulicaria gabrielii
and Iphionopsis oblanceolata (Compositae, Inuleae), two new species
from NE Somalia. – Willdenowia 29: 115-121.
Kilian N. 1999b. Studies in the Compositae of
the Arabian Peninsula and Socotra 1. Pulicaria gamal-eldinae sp. nova
(Inuleae) bridges the gap between Pulicaria and former
Sclerostephane (now P. sect. Sclerostephane). –
Willdenowia 29: 167-185.
Kilian N. 1999c. Studies in the Compositae of
the Arabian Peninsula and Socotra 2. Pulicaria samhanensis sp. nova
(Inuleae) from Dhofar and notes on other S Arabian species of the genus. –
Willdenowia 29: 187-196.
Kilian N, Gemeinholzer B. 2007. Studies in
the Compositae of the Arabian Peninsula and Socotra 7. Erythroseris, a
new genus and the previously unknown sister group of Cichorium
(Cichorieae subtribe Cichoriinae). – Willdenowia 37: 283-296.
Kilian N, Hein P. 1999. Studies in the
Compositae of the Arabian Peninsula and Socotra 2. Pulicaria
samhanensis sp. nova (Inuleae) from Dhofar and notes on other S Arabian
species of the genus. – Willdenowia 29: 187-196.
Kilian N, Miller AG. 2000. Studies in the
Compositae of the Arabian Peninsula and Socotra 5. Distephanus qazmi
(Vernonieae, Gymnantheminae), a remarkable new species from the island of
Socotra, Yemen. – Willdenowia 30: 83-91.
Kilian N, Oberprieler C, Vogt R.
1995.Chromosome numbers of North African phanerogams V. Some counts in
Launaea (Compositae, Lactuceae). – Willdenowia 25: 273-281.
Kilian N, Gemeinholzer B, Lack HW. 2009.
Cichorieae. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 343-383.
Kilian N, Sennikov A, Wang Z-H, Gemeinholzer
B, Zhang J-W. 2017. Sub-Paratethyan origin and Middle to Late Miocene principal
diversification of the Lactucinae (Compositae: Cichorieae) inferred from
molecular phylogenetics, divergence-dating and biogeographic analysis. –
Taxon 66: 675-703.
Kilian N, Galbany-Casals M, Sommerer R,
Oberprieler C, Smissen R, Miller A, Rabe K. 2017. Systematics of
Libinhania, a new endemic genus of Gnaphalieae (Asteraceae) from the Socotra
archipelago (Yemen), inferred from plastid, low-copy nuclear and nuclear
ribosomal DNA loci. – Bot. J. Linn. Soc. 183: 373-412.
Kilian N, Hand R, Hadjikyriakou GN,
Christodoulou CS, Dagher-Kharrat MB. 2017. Astartoseris
(Cichorieae, Asteraceae), a new,
systematically isolated monospecific genus accommodating Lactuca
triquetra endemic to Lebanon and Cyprus. – Willdenowia 47: 115-125.
Kim H-G, Keeley SC, Vroom PS, Jansen RK.
1998. Molecular evidence for an African origin of the Hawaiian endemic
Hesperomannia (Asteraceae). – Proc. Natl. Acad.
Sci. U.S.A. 95: 15440-15445.
Kim H-G, Loockerman DJ, Jansen RK. 2002.
Systematic implications of ndhF sequence variation in the Mutisieae
(Asteraceae). – Syst. Bot. 27:
598-609.
Kim H-G, Funk VA, Vlasek A, Zimmer E. 2003. A
phylogeny of the Munnoziinae (Compositae, Liabeae): circumscription of
Munnozia and a new placement of M. perfoliata. – Plant
Syst. Evol. 239: 171-185.
Kim I. 1987. Comparative anatomy of some
parents and hybrids of the Hawaiian Madiinae (Asteraceae). – Amer. J. Bot. 74:
1224-1238.
Kim K-J, Jansen RK. 1992. Phylogenetic and
evolutionary implications of interspecific chloroplast DNA variation in
Krigia (Lactuceae-Asteraceae). – Syst. Bot. 17:
449-469.
Kim K-J, Jansen RK. 1994. Comparisons of
phylogenetic hypotheses among different data sets in dwarf dandelions
(Krigia, Asteraceae):
additional information from internal transcribed spacer sequences of nuclear
ribosomal DNA. – Plant Syst. Evol. 190: 157-185.
Kim K-J, Jansen RK. 1995. ndhF
sequence evolution and the major clades in the sunflower family. – Proc.
Natl. Acad. Sci. U.S.A. 92: 10379-10383.
Kim K-J, Mabry TJ. 1991. Phylogenetic and
evolutionary implications of nuclear ribosomal DNA variation in dwarf
dandelions (Krigia, Lactuceae, Asteraceae). – Plant Syst. Evol.
177: 53-69.
Kim K-J, Turner BL, Jansen RK. 1992.
Phylogenetic and evolutionary implications of interspecific chloroplast DNA
variation in Krigia (Asteraceae-Lactuceae). – Syst. Bot.
17: 449-469.
Kim K-J, Jansen RK, Wallace RS, Michaels HJ,
Palmer JD. 1992. Phylogenetic implications of rbcL sequence variation
in the Asteraceae. – Ann.
Missouri Bot. Gard. 79: 428-445.
Kim K-J, Choi K-S, Jansen RK. 2005. Two
chloroplast DNA inversions originated simultaneously during the early evolution
of the sunflower family (Asteraceae). – Mol. Biol. Evol. 22:
1783-1792.
Kim S-C, Crawford DJ, Jansen RK. 1996.
Phylogenetic relationships among the genera of the subtribe Sonchinae (Asteraceae): evidence from ITS
sequences. – Syst. Bot. 21: 417-432.
Kim S-C, Crawford DJ, Francisco-Ortega J,
Santos-Guerra A. 1996. A common origin for woody Sonchus and five
related genera in the Macaronesian islands: molecular evidence for extensive
radiation. – Proc. Natl. Acad. Sci. U.S.A. 93: 7743-7748.
Kim S-C, Crawford DJ, Jansen RK,
Santos-Guerra A. 1999. The use of a non-coding region of chloroplast DNA in
phylogenetic studies of the subtribe Sonchinae (Asteraceae: Lactuceae). – Plant
Syst. Evol. 215: 85-99.
Kim S-C, Crawford DJ, Francisco-Ortega J,
Santos-Guerra A. 1999. Adaptive radiation and genetic differentiation in the
woody Sonchus alliance (Asteraceae: Sonchinae) in the Canary
Islands. – Plant Syst. Evol. 215: 101-118.
Kim S-C, Crawford DJ, Tadesse M, Berbee M,
Ganders FR, Pirseyedi M, Esselman EJ. 1999. ITS sequences and phylogenetic
relationships in Bidens and Coreopsis (Asteraceae). – Syst. Bot. 24:
480-493.
Kim S-C, Lu CT, Lepschi BJ. 2004.
Phylogenetic positions of Actites megalocarpa and Sonchus
hydrophilus (Sonchinae: Asteraceae) based on ITS and
chloroplast non-coding DNA sequences. – Aust. Syst. Bot. 17: 73-81.
Kim S-C, Lee C-G, Mejías JA. 2007.
Phylogenetic analysis of chloroplast matK gene and ITS of nrDNA
sequences reveals polyphyly of the genus Sonchus and new relationships
among the subtribe Sonchinae (Asteraceae: Cichorieae). – Mol.
Phylogen. Evol. 44: 578-597.
Kimball RT, Crawford DJ. 2004. Phylogeny of
Coreopsideae (Asteraceae) using
ITS sequences suggests lability in reproductive characters. – Mol. Phylogen.
Evol. 33: 127-139.
King BL. 1986. A systematic survey of the
leaf flavonoids of Lychnophora (Asteraceae: Vernonieae). – Syst.
Bot. 11: 403-414.
King RM. 1965. Chromosome numbers of Thailand
Compositae. – Phytologia 11: 217-218.
King RM, Dawson HW. 1975. Cassini on
Compositae 1-3. – Oriole editions, New York.
King RM, Krantz VE. 1975. Ultraviolet
reflectance patterns in the Asteraceae I. Local and cultivated
species. – Phytologia 31: 66-114.
King RM, Robinson H. 1966. Generic
limitations in the Hofmeisteria complex (Compositae-Eupatorieae). –
Phytologia 12: 465-475.
King RM, Robinson H. 1969. Studies in the
Eupatorieae (Compositae) XVI. A monograph of the genus Decachaeta. –
Brittonia 21: 285.
King RM, Robinson H. 1970a. Studies in the
Eupatorieae (Compositae) XIX. New combinations in Ageratina. –
Phytologia 19: 208-229.
King RM, Robinson H. 1970b. Studies in the
Eupatorieae (Compositae) XXIV. A new genus, Stomatanthes. –
Phytologia 19: 429-430.
King RM, Robinson H. 1970c. Studies in the
Eupatorieae (Compositae) XXIX. The genus Chromolaena. – Phytologia
20: 196-209.
King RM, Robinson H. 1970d. The new
synantherology. – Taxon 19: 6-11.
King RM, Robinson H. 1970e. Studies in the
Eupatorieae (Compositae) XXXI. A new genus, Polyanthina. –
Phytologia 20: 213.
King RM, Robinson H. 1970f.
Eupatorium, a composite genus of Arcto-Tertiary distribution. –
Taxon 19: 769-771.
King RM, Robinson H. 1971a. Studies in the
Eupatorieae (Compositae) XXXVI. A new genus, Neobartlettia. –
Phytologia 21: 294-297.
King RM, Robinson H. 1971b. Studies in the
Eupatorieae (Asteraceae) XXXVII.
The genus Hebeclinium. – Phytologia 21: 298-301.
King RM, Robinson H. 1971c. Studies in the
Eupatorieae (Asteraceae) LIX. A
new genus, Steviopsis. – Phytologia 22: 156-157.
King RM, Robinson H. 1971d. Studies in the
Eupatorieae (Asteraceae) LXI.
Additions to the Hebeclinium complex with Bartlettina, a new
generic name. – Phytologia 22: 160-162.
King RM, Robinson H. 1972a. Studies in the
Eupatorieae (Asteraceae) LXVIII. A
new genus, Conocliniopsis. – Phytologia 23: 307-309.
King RM, Robinson H. 1972b. Studies in the
Eupatorieae (Asteraceae) LXXI. A
new genus, Hatschbachiella. – Phytologia 23: 393-394.
King RM, Robinson H. 1972c. Studies in the
Eupatorieae (Asteraceae) LXXVII.
Additions to the genus Steviopsis. – Phytologia 24: 60-62.
King RM, Robinson H. 1972d. Studies in the
Eupatorieae (Asteraceae) LXXXVII.
The genus Alomia. – Phytologia 24: 108-111.
King RM, Robinson H. 1972e. Studies in the
Eupatorieae (Asteraceae) LXXXVIII.
Additions to the genus Ageratum. – Phytologia 24: 112-117.
King RM, Robinson H. 1972f. Studies in the
Eupatorieae (Asteraceae) CII. A
new genus Condylidium. – Phytologia 24: 380-381.
King RM, Robinson H. 1973. Studies in the
Eupatorieae (Asteraceae) CXIII. A
new genus, Matudina. – Phytologia 26: 170-173.
King RM, Robinson H. 1974. Studies in the
Eupatorieae (Asteraceae) CXVIII.
New species of Ageratum, Fleischmannia and
Hebeclinium from northern South America. – Phytologia 31:
311-316.
King RM, Robinson H. 1975a. Studies in the
Eupatorieae (Asteraceae) CXLVII.
Additions to the genera Amboroa, Ayapanopsis, and
Hebeclinium in South America. – Phytologia 31: 311-316.
King RM, Robinson H. 1975b. Studies in the
Eupatorieae (Asteraceae) CLVI.
Various new combinations. – Phytologia 32: 283-285.
King RM, Robinson H. 1977a. Eupatorieae – a
systematic review. – In: Heyxood VH, Harborne JB, Turner BL (eds), Biology
and chemistry of the Compositae 1, Academic Press, London.
King RM, Robinson H. 1977b. Guayania
davidsei and Hebeclinium gentryi. New species from northern South
America (Eupatorieae-Asteraceae).
– Ann. Missouri Bot. Gard. 64: 366-370.
King RM, Robinson H. 1978. Studies in the
Eupatorieae (Asteraceae) CLXVIII.
Additions to the genus Ageratina. – Phytologia 38: 323-355.
King RM, Robinson H. 1979. Studies in the
Eupatorieae (Asteraceae) CLXXVI.
The relationship of Eupatorium cyrili-nelsonii. – Phytologia 44:
84-87.
King RM, Robinson H. 1980a. Studies in the
Eupatorieae (Asteraceae) CXCII.
Validation of subtribes. – Phytologia 46: 446-450.
King RM, Robinson H. 1980b. Studies in the
Eupatorieae (Asteraceae) CXCVII.
Three additions to Bartlettina. – Phytologia 47: 121-125.
King RM, Robinson H. 1987. The genera of the
Eupatorieae (Asteraceae). –
Missouri Bot. Gard. Monogr. Syst. Bot. 22: 1-581.
King RM, Robinson H. 1995. Generic limits in
the Alomiinae (Eupatorieae-Asteraceae), and new combinations in
Brickelliastrum and Barroetea. – Phytologia 78: 124-126.
King RM, Kyhos DW, Powell AM, Raven PH,
Robinson H. 1976. Chromosome numbers in Compositae XIII. Eupatorieae. – Ann.
Missouri Bot. Gard. 63: 862-888.
Kingham DL. 1976. A study of the pollen
morphology of the tropical African and certain other Vernonieae (Compositae).
– Kew Bull. 31: 9-26.
King-Jones S. 1999. Studies in the Compositae
of the Arabian Peninsula and Socotra 4. The Arabian species of Pluchea
(Compositae, Plucheeae). – Willdenowia 29: 203-220.
King-Jones S, Kilian N. 1999. Studies in the
Compositae of the Arabian Peninsula and Socotra 3. Pluchea aromatica
from Socotra is actually a species of Pulicaria (Inuleae). –
Willdenowia 29: 197-202.
Kirkman KL. 1981. Taxonomic revision of
Centratherum and Phyllocephalum (Compositae; Vernonieae). –
Rhodora 83: 1-23.
Kirschner J. 1996. A nomenclatural and
taxonomic account of Willemetia (Compositae, Lactuceae, Crepidinae).
– Taxon 45: 627-630.
Kirschner J, Štěpánek. 1998. A monograph
of Taraxacum section Palustria. – Institute of Botany,
Academy of Sciences of the Czech Republic, Průhonice.
Kirschnerová L, Kirschner J. 1996. A
nomenclatural and taxonomic account of Willemetia (Compositae,
Lactuceae, Crepidinae). – Taxon 45: 627-630.
Kita Y, Fujikawa K, Ito M, Ohba H, Kato M.
2004. Molecular phylogenetic analyses and systematics of the genus
Saussurea and related genera (Asteraceae, Cardueae). – Taxon 53:
679-690.
Kitamura S. 1938. Leibnitzia. – J.
Jap. Bot 14: 296-297.
Kitamura S. 1969. Compositae of Southeast
Asia and Himalayas IV. – Acta Phytotaxon. Geobot. 24: 1-27.
Klaassen ES, Kwembeya EG, Maass E. 2009. A
taxonomic revision of the genus Pentatrichia (Asteraceae). – South Afr. J. Bot.
75: 153-164.
Klásek A, Sedmera P, Boeva A, Šantavý F.
1971. Pyrrolizidine alkaloids XVI. Alkaloids from some plants of the genus
Ligularia. – Coll. Czech. Chem. Commun. 36: 2205-2215.
Klásek A, Sedmera P, Boeva A, Šantavý F,
1973. Pyrrolizidine alkaloids XX. Nemorensine, an alkaloid from Senecio
nemorensis L. – Coll. Czech. Chem. Commun. 38: 2504-2512.
Klokov MV, Krytzka LI. 1984. A system of the
genera Ptarmica Mill. and Achillea L. – Ukrayins’k. Bot.
Žurn. 41: 1-11. [In Ukrainian]
Kneissl B. 1981. Karpologische Untersuchung
an Anthemideae. – Zulassungsarbeit, München, Germany.
Knox EB.1996. What is the origin of the giant
Senecios in eastern Africa? – In: Hind DJN, Beentje HJ (eds), Compositae:
systematics. Proceedings of the International Compositae Conference, Kew, 1994,
I, Royal Botanic Gardens, Kew, pp. 691-703.
Knox EB, Li C. 2017. The East Asian origin of
the giant lobelias. – Amer. J. Bot. 104: 924-938.
Knox EB, Friedrich E. 1974. Tetrad pollen
grain development and sterility in Leschenaultia formosa (Goodeniaceae). – New Phytol. 73:
251-258.
Knox EB, Kowal RR. 1993. Chromosome numbers
of the East african giant senecios and giant lobelias and their evolutionary
significance. – Amer. J. Bot. 80: 847-853.
Knox EB, Palmer JD. 1995a. The origin of
Dendrosenecio within the Senecioneae (Asteraceae) based on plastid DNA
evidence. – Amer. J. Bot. 82: 1567-1573.
Knox EB, Palmer JD. 1995b. Chloroplast DNA
variation and the recent radiation of the giant senecios (Asteraceae) on the tall mountains of
eastern Africa. – Proc. Natl. Acad. Sci. U.S.A. 92: 10349-10353.
Knox EB, Palmer JD. 1998. Chloroplast DNA
evidence on the origin and radiation of the giant lobelias in eastern Africa.
– Syst. Bot. 23: 109-149.
Knox EB, Palmer JD. 1999. The chloroplast
genome of Lobelia thuliniana (Lobeliaceae): expansion of the inverted
repeat in an ancestor of the Campanulales. – Plant Syst. Evol.
214: 49-64.
Knox EB, Downie SR, Palmer JD. 1993.
Chloroplast genome rearrangements and the evolution of giant lobelias from
herbaceous ancestors. – Mol. Biol. Evol. 10: 414-430.
Knox EB, Muasya AM, Phillipson PB. 2006. The
Lobeliaceae originated in southern Africa. – In: Ghazanfar SA, Beentje HJ
(eds), Taxonomy and ecology of African plants, their conservation and
sustainable use, Proceedings of the 17th AETFAT Congress, Addis
Abeba, Ethiopia, Royal Botanic Gardens, Kew, pp. 215-227.
Knox EB, Muasya AM, Muchhala N. 2008. The
predominantly South American clade of Lobeliaceae. – Syst. Bot. 33:
462-468.
Kobayashi M, Horikawa S, Degrandi IH, Ueno J,
Mitsuhashi H. 1977. Dulcosides A and B, new diterpene glycosides from
Stevia rebaudiana. – Phytochemistry 16: 1405-1408.
Koch MF. 1930a. Studies in the anatomy and
morphology of the Compositae flower I. The corolla. – Amer. J. Bot. 17:
938-952.
Koch MF. 1930b. Studies in the anatomy and
morphology of the Compositae flower II. The corollas of the Heliantheae and
Mutisieae. – Amer. J. Bot. 17: 995-1010.
Kochjarova J. 1997. Nacrt taxonomickej
problematiky rodu Tephroseris v Zapadnych Karpatoch. – Preslia 69:
71-93.
Kohda H, Tanaka O, Nishi K. 1976. Diterpene
glycosides of Stevia paniculata. – Chem. Pharm. Bull. 24:
1040-1044.
Kolakovsky AA. 1986. Carpology of the Campanulaceae and problems of
taxonomy. – Bot. Žurn. 71: 1155-1168. [In Russian with English summary]
Kolakovsky AA. 1987. System of the Campanulaceae family from the Old
World. – Bot. Žurn. 72: 1572-1579. [In Russian]
Kolakovsky AA. 1994. The conspectus of the
system of the Old World Campanulaceae. – Bot. Žurn. 79:
109-124. [In Russian]
Koller D, Roth N. 1964. Studies on the
ecological and physiological significance of amphicarpy in Gymnarrhena
micrantha (Compositae). – Amer. J. Bot. 51: 26-35.
Kolokoto R, Magee AR. 2018. Cape stinkweeds:
taxonomy of Oncosiphon (Anthemideae, Asteraceae). – South Afr. J. Bot.
117: 57-70.
Konishi N, Watanabe K, Kosuge K. 2000.
Molecular systematics of Australian Podolepis (Asteraceae: Gnaphalieae): evidence
from DNA sequences of the nuclear ITS region and the chloroplast matK
gene. – Aust. Syst. Bot. 13: 709-727.
Koontz JA, Soltis DE. 1999. DNA sequence data
reveal polyphyly of Brexioideae (Brexiaceae; Saxifragaceae
sensu lato). – Plant Syst. Evol. 219: 199-208.
Koontz JA, Lundberg J, Soltis DE. 2006
[2007]. Rousseaceae. – In:
Kubitzki K, Kadereit JW, Jeffrey C (eds), The families and genera of vascular
plants VIII. Flowering plants. Eudicots. Asterales, Springer, Berlin,
Heidelberg, New York, pp. 611-613.
Koopman MM, Ayers TJ. 2005. Nectar spur
evolution in the Mexican lobelias (Campanulaceae: Lobelioideae). –
Amer. J. Bot. 92: 558-562.
Koopman WJM, Guetta E, Wiel CCM van de,
Vosman B, Berg RG van den. 1998. Phylogenetic relationships among
Lactuca (Asteraceae)
species and related genera based on ITS-1 DNA sequences. – Amer. J. Bot. 85:
1517-1530.
Kopriva S, Chu C-C, Bauwe H. 1996. Molecular
phylogeny of Flaveria as deduced from the analysis of nucleotide
sequences encoding the H-protein of the glycine cleavage system. – Plant,
Cell and Environment 19: 1028-1036.
Kornkven AB, Watson LE, Estes JR. 1998.
Phylogenetic analysis of Artemisia sect. Tridentatae (Asteraceae) based on sequences from
internal transcribed spacers (ITS) of nuclear ribosomal DNA. – Amer. J. Bot.
85: 1787-1795.
Kornkven AB, Watson LE, Estes JR. 1999.
Molecular phylogeny of Artemisia sect. Tridentatae (Asteraceae) based on chloroplast DNA
restriction site variation. – Syst. Bot. 24: 69-84.
Koster JT, 1966. The Compositae of New Guinea
I. – Nova Guinea 24: 524-612.
Koster JT. 1972. The Compositae of New Guinea
III. – Blumea 20: 193-226.
Koutsovoulou K, Daws MI, Thanos CA. 2014. Campanulaceae: a family with small
seeds that require light for germination. – Ann. Bot. 113: 135-143.
Koul V, Singh D. 1982. Cytoembryological
studies on Indian Taraxacum Weber I. Microsporangium,
microsporogenesis and microgametogenesis. – Cytologia 47: 125-135.
Kovanda M. 1968. New taxa and combinations in
the subsection Heterophylla (Witas) Fed. of the genus
Campanula L. – Folia Geobot. Phytotaxon. 3: 407-411.
Kovanda M. 1970a. Polyploidy and variation in
the Campanula rotundifolia complex I (General). – Rozpr. Českoslov.
Akad. Ved. 80: 1-95.
Kovanda M. 1970b. Polyploidy and variation in
the Campanula rotundifolia complex II (Taxonomy). 1. Revision of the
groups Saxicolae, Lanceolatae and Alpicolae in
Czechoslovakia and adjacent regions. – Folia Geobot. Phytotaxon. 5:
171-208.
Kovanda M. 1977. Polyploidy and variation in
the Campanula rotundifolia complex 2 (Taxonomy). 2. Revision of the
groups Vulgares and Scheuchzerianae in Czechoslovakia and
adjacent regions. – Folia Geobot. Phytotaxon. 12: 23-89.
Kovačić S. 2004. The genus
Campanula L. (Campanulaceae) in Croatia,
circum-Adriatic and West Balkan region. – Acta Bot. Croat. 63: 171-202.
Kowal RR. 1975. Systematics of Senecio
aureus and allied species in the Gaspé Peninsula, Quebec. – Mem. Torrey
Bot. Club 23: 1-113.
Koyama H. 1965. On the chromosome number of
Senecio nikoensis. – Acta Phytotaxon. Geobot. 21: 132.
Koyama H. 1967. Taxonomic studies on the
tribe Senecioneae of Eastern Asia I. General part. – Mem. Fac. Sci. Kyoto
Univ., Ser. Biol. 33: 181-209.
Koyama H. 1969. Taxonomic studies on the
tribe Senecioneae of East Asia II. Enumeration of the species of eastern Asia.
– Mem. Fac. Sci. Kyoto Univ., Ser. Biol. 2: 19-59, 137-183.
Koyama H. 2004. Taxonomic studies in the
Compositae of Thailand 16. Vernonia sects. Xipholepis and
Claotrachelas. – Bull. Natl. Sci. Mus. Tokyo, Ser. B, 30: 21-34.
Koyama H. 2005. Taxonomic studies in the
Compositae of Thailand 17: Vernonia Sect. Lepidaploa Subsect.
Paniculatae. – Bull. Natl. Sci. Mus. Tokyo, Ser. B, 31: 67-78.
Kozo-Poljanski BM. 1923. On the systematic
position of the family Compositae. – J. Russ. Bot. Soc. 8: 167-191.
Krach B. 1988. Tephroseris
integrifolia subsp. vindelicorum – eine neue Sippe vom
Augsburger Lechfield. – Mitt. Bot. Staatssamml. München 27: 73-86.
Krach JE. 1976. Samenanatomie der Rosifloren
I. Die Samen der Saxifragaceae.
– Bot. Jahrb. Syst. 97: 1-60.
Krach JE. 1977. Seed characters in and
affinities among the Saxifragaceae.
– Plant Syst. Evol. [Suppl.] 1: 141-153.
Krebs HC, Ramiarantsoa H. 1998. Piperidine
alkaloids and other constituents of Dialypetalum floribundum. –
Phytochemistry 48: 911-913.
Ku MSB, Monson RK, Littlejon RO Jr, Nakamoto
H, Fisher DB, Edwards GE. 1983. Photosynthetic characteristics of
C3-C4 intermediate Flaveria species. – Plant
Physiol. 71: 944-948.
Kubitzki K, Kadereit JW, Jeffrey C (eds).
2006 [2007]. The families and genera of vascular plants VIII. Flowering plants.
Eudicots. Asterales. – Springer, Berlin, Heidelberg, New York.
Kuprianova LA, Tscherneva OV. 1982. Pollen
morphology and ultrastructure of palynoderma in the species of the genus
Cousinia (Asteraceae) in
relation to the systemarics of the genus. – Bot. Žurn. 67: 581-589.
Kyhos DW. 1965. The independent aneuploid
origin of two species of Chaenactis (Compositae) from a common
ancestor. – Evolution 19: 26-43.
Kyhos DW, Carr GD, Baldwin BG. 1990.
Biodiversity and cytogenetics of the tarweeds (Asteraceae: Heliantheae-Madiinae).
– Ann. Missouri Bot. Gard. 77: 84-95.
Kynčlová M. 1970. Comparative morphology of
achenes of the tribe Anthemideae Cass. (Family Asteraceae) and its taxonomic
significance. – Preslia 42: 33-53.
Lack HW. 1975. Die Gattung Picris
L., sensu lato, im ostmediterran-westasiatischen Raum. – Ph.D. diss.,
Universität Wien, Austria.
Lack HW. 1978. Die Gattung
Heywoodiella Svent. & Bramw. (Asteraceae, Lactuceae). –
Willdenowia 8: 329-339.
Lack HW. 1979a. The subtribe Hypochoeridinae
(Asteraceae, Lactuceae) in the
tropics and the southern hemisphere. – In: Larsen K, Holm-Nielsen LB (eds),
Tropical botany, Academic Press, London, pp. 265-276.
Lack HW. 1979b. The genus Picris (Asteraceae, Lactuceae) in tropical
Africa. – Plant Syst. Evol. 131: 35-52.
Lack HW. 1979c. New species of
Picris (Asteraceae,
Lactuceae) from Australia. – Phytologia 42: 209-214.
Lack HW. 1981. Die Lactuceae (Asteraceae) der Azorischen Inseln.
– Willdenowia 11: 211-247.
Lack HW, Leuenberger B. 1979. Pollen and
taxonomy of Urospermum (Asteraceae, Lactuceae). – Pollen
Spores 21: 415-425.
Lacombe L, Langlois N, Das BC, Potier P.
1970. Plants from New Caledonia IV. Alkaloids of Phelline comosa
Labill. (Ilicaceae). – Bull. Soc. Chim. Fr. 10: 3535-3543.
Laduke JC. 1982a. A new species of
Tithonia and infrageneric classification. – Rhodora 84: 139-141.
Laduke JC. 1982b. Revision of
Tithonia. – Rhodora 84: 453-522.
Laduke JC. 1982c. Flavonoid chemistry and
systematics of Tithonia (Compositae). – Amer. J. Bot. 69:
784-792.
Lagomarsino LP, Antonelli A, Muchhala N,
Timmermann A, Mathews S, Davis CC. 2014. Phylogeny, classification, and fruit
evolution of the species-rich Neotropical bellflowers (Campanulaceae: Lobelioideae). –
Amer. J. Bot. 101: 2097-2112.
Lakušić D, Conti F. 2004. Asyneuma
pichleri (Campanulaceae),
a neglected species of the Balkan Peninsula. – Plant Syst. Evol. 247:
23-36.
Lakušić D, Liber Z, Nikolić T, Surina B,
Kovačić S, Bogdanović S, Stefanović S. 2013. Molecular phylogeny of the
Campanula pyramidalis species complex (Campanulaceae) inferred from
chloroplast and nuclear non-coding sequences and its taxonomic implications.
– Taxon 62: 505-524.
Lamboy WF. 1988. The status of Aster
commixtus and a new species of Aster from the southeastern United
States. – Syst. Bot. 13: 187-195.
Lammers TG. 1988a. Chromosome numbers and
their systematic implications in the Hawaiian Lobelioideae (Campanulaceae). – Amer. J. Bot.
75: 1130-1134.
Lammers TG. 1988b. New taxa, new names, and
new combinations in the Hawaiian Lobelioideae (Campanulaceae). – Syst. Bot. 13:
496-508.
Lammers TG. 1989. Revision of
Brighamia (Campanulaceae: Lobelioideae) a
caudiciform succulent endemic to the Hawaiian Islands. – Syst. Bot. 14:
133-138.
Lammers TG. 1990. Sequential paedomorphosis
among the endemic Hawaiian Lobelioideae (Campanulaceae). – Taxon 39:
206-211.
Lammers TG. 1991. Systematics of
Clermontia (Campanulaceae: Lobelioideae). –
Syst. Bot. Monogr. 32: 1-94.
Lammers TG. 1992. Circumscription and
phylogeny of the Campanulales.
– Ann. Missouri Bot. Gard. 79: 388-413.
Lammers TG. 1993. Chromosome numbers of Campanulaceae III. Review and
integration of data for subfamily Lobelioideae. – Amer. J. Bot. 80:
660-675.
Lammers TG. 1995a. Patterns of speciation and
biogeography in Clermontia (Campanulaceae, Lobelioideae). –
In: Wagner WL, Funk V (eds), Hawaiian biogeography: evolution on a hot spot
archipelago, Smithsonian Inst. Press, Washington, D.C., pp. 338-362.
Lammers TG. 1995b. Transfer of the southern
African species of Lightfootia, nom. illeg., to Wahlenbergia
(Campanulaceae,
Campanuloideae). – Taxon 44: 333-339.
Lammers TG. 1996. Phylogeny, biogeography,
and systematics of the Wahlenbergia fernandeziana complex (Campanulaceae: Campanuloideae).
– Syst. Bot. 21: 397-415.
Lammers TG. 1998a. Nemacladoideae, a new
subfamily of Campanulaceae. –
Novon 8: 36-37.
Lammers TG. 1998b. Review of the Neotropical
endemics Burmeistera, Centropogon, and Siphocampylus
(Campanulaceae: Lobelioideae),
with description of 18 new species and a new section. – Brittonia 50:
233-262.
Lammers TG. 1999. Nomenclatural consequences
of the synonymization of Hypsela reniformis (Campanulaceae: Lobelioideae). –
Novon 9: 73-76.
Lammer TG. 2000. Revision of Lobelia
sect. Tupa (Campanulaceae: Lobelioideae). –
Sida 19: 87-110.
Lammers TG. 2002. Seventeen new species of
Lobelioideae (Campanulaceae)
from South America. – Novon 12: 206-233.
Lammers TG. 2004. Revision of
Lobelia sect. Homochilus (Campanulaceae: Lobelioideae). –
Sida 21: 591-623.
Lammers TG. 2005. Revision of
Delissea (Campanulaceae: Lobelioideae). –
Syst. Bot. Monogr. 73: 1-75.
Lammers TG. 2006a [2007]. Campanulaceae. – In: Kubitzki K,
Kadereit JW, Jeffrey C (eds), The families and genera of vascular plants VIII.
Flowering plants. Eudicots. Asterales, Springer, Berlin, Heidelberg, New York,
pp. 26-56.
Lammers TG. 2006b [2007]. Pentaphragmataceae. – In:
Kubitzki K, Kadereit JW, Jeffrey C (eds), The families and genera of vascular
plants VIII. Flowering plants. Eudicots. Asterales, Springer, Berlin,
Heidelberg, New York, pp. 605-607.
Lammers TG. 2007. World checklist and
bibliography of Campanulaceae.
– Royal Botanic Gardens, Kew.
Lammers TG. 2009. Revision of the endemic
Hawaiian genus Trematolobelia (Campanulaceae: Lobelioideae). –
Brittonia 61: 126-143.
Lammers TG. 2011. Revision of the
infrageneric classification of Lobelia L. (Campanulaceae: Lobelioideae). –
Ann. Missouri Bot. Gard. 98: 37-62.
Lammers TG, Freeman CE. 1986. Ornithophily
among the Hawaiian Lobelioideae (Campanulaceae): evidence from
floral nectar sugar composition. – Amer. J. Bot. 73: 1613-1619.
Lammers TG, Hensold N. 1992. Chromosome
numbers of Campanulaceae II.
The Lobelia tupa complex of Chile. – Amer. J. Bot. 79: 585-588.
Lammers TG, Klein LL. 2010. A synopsis of
Codonopsis subg. Pseudocodonopsis (Campanulaceae: Campanuloideae),
with description of a new species of uncertain provenance. – Bot. Stud. 51:
553-561.
Lamont E. 2004. New combinations in
Eutrochium (Asteraceae:
Eupatorieae), an earlier name than Eupatoriadelphus. – Sida 21:
901-902.
Lander NS. 1976. Actites, a new
genus of Compositae from Australia. – Telopea 1: 129-135.
Lander NS. 1989. Apostates (Asteraceae, Astereae), a new genus
from the south-eastern Polynesian island of Rapa. – Aust. Syst. Bot. 2:
129-133.
Lander NS. 1991. New taxa and new
combinations in Olearia (Asteraceae: Astereae) from
south-eastern Australia. – Telopea 4: 145-164.
Lander NS. 2013. Pilbara, a new
genus of Asteraceae (tribe
Astereae) from Western Australia. – Nuytsia 23: 117-123.
Lander NS, Barry R. 1980a. Reinstatement of
the genus Kippistia F. Muell. (Asteraceae-Astereae). – Nuytsia 3:
215-219.
Lander NS, Barry R. 1980b. A review of the
genus Minuria DC. (Asteraceae-Astereae). – Nuytsia 3:
221-237.
Lander NS, Hurter PJH. 2013.
Pleurocarpaea gracilis (Asteraceae: Vernonieae), a new
species from the Pilbara region of Western Australia. – Nuytsia 23:
109-115.
Lane MA. 1979. Taxonomy of the genus
Amphiachyris (Asteraceae-Astereae). – Syst. Bot.
4: 178-189.
Lane MA. 1980. Systematics of
Amphiachyris, Greenella, Gutierrezia,
Gymnosperma, Thurovia and Xanthocephalum
(Compositae: Astereae). – Ph.D. diss., University of Texas, Austin, Texas.
Lane MA. 1982. Generic limits of
Xanthocephalum, Gutierrezia, Amphiachyris,
Gymnosperma, Greenella, and Thurovia
(Compositae-Astereae). – Syst Bot. 7: 405-416.
Lane MA. 1983. Taxonomy of
Xanthocephalum (Compositae-Astereae). – Syst. Bot. 8: 305-316.
Lane MA. 1985. Taxonomy of
Gutierrezia (Compositae-Astereae) in North America. – Syst. Bot. 10:
7-28.
Lane MA. 1988. Generic relationships and
taxonomy of Acamptopappus (Compositae: Astereae). – Madroño 35:
247-265.
Lane MA. 1996. Pollination biology of
Compositae. – In: Caligari P, Hind DJN (eds), Compositae: Biology and
utilization. Proceedings of the International Compositae Conference, Kew, 1994,
vol. 2, Royal Botanic Gardens, Kew, pp. 61-80.
Lane MA, Hartman RL. 1996. Reclassification
of North American Haplopappus (Compositae: Astereae) completed:
Rayjacksonia gen. nov. – Amer. J. Bot. 83: 356-370.
Lane MA, Morgan DR, Suh Y-B, Simpson BB,
Jansen RK. 1996. Relationships of North American genera of Astereae, based on
chloroplast DNA restriction site data. – In: Hind DJN, Beentje Hj (eds),
Compositae: systematics. Proceedings of the International Compositae
Conference, Kew, 1994, vol. 1, Royal Botanic Gardens, Kew, pp. 49-77.
Langlet O. 1936. Några bidrag till
kännedomen om kromosomtalen inom Nymphaeaceae, Ranunculaceae, Polemoniaceae, och
Compositae. – Svensk Bot. Tidskr. 30: 288-294.
Langlois N. 1990. New homoerythrinane
alkaloids from Phelline spp. – Heterocycles (Tokyo) 30: 659-664.
Langlois N, Razafimbelo J. 1988. Alcaloïdes
de Phelline sp. aff. Phelline lucida: origine de l’holidine
et du phellinamide; configuration de la comosidine, de la lucidinine, et de
leurs derives. – J. Natur. Prod. 51: 499-503.
Larson D. 2007. Reproduction strategies in
introduced Nymphoides peltata populations revealed by genetic markers.
– Aquatic Bot. 86: 402-406.
Launert E. 1983. 98. Goodeniaceae. – In:Launert E
(ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London,
pp. 85-87.
Laurent N, Bremer B, Bremer K. 1998.
Phylogeny and generic interrelationships of the Stylidiaceae (Asterales), with a
possible extreme case of floral paedomorphosis. – Syst. Bot. 23: 289-304.
Lavialle P. 1912. Recherches sur le
développement de l’ovaire en fruit chez les Composées. – Ann. Sci. Nat.
Bot., 9th ser., 15: 39-152.
Lawton-Rauh A, Robichaux RH, Purugganan MD.
2003. Patterns of nucleotide variation in homoeologous regulatory genes in the
allotetraploid Hawaiian silversword alliance (Asteraceae). – Mol. Ecol. 12:
1301-1313.
Lee C, Kim S-C, Lundy K, Santos-Guerra A.
2005. Chloroplast DNA phylogeny of the woody Sonchus alliance (Asteraceae: Sonchinae) in the
Macaronesian Islands. – Amer. J. Bot. 92: 2072-2085.
Lee J, Baldwin BG, Gottlieb LD. 2003.
Phylogenetic relationships among the primarily North American genera of
Cichorieae (Compositae) based on analysis of 18S-26S nuclear rDNA ITS and ETS
sequences. – Syst. Bot. 28: 616-626.
Lee YS. 1981. Serological investigations in
Ambrosia (Compositae: Ambrosieae) and relatives. – Syst. Bot. 6:
113-125.
Leenhouts PW. 1957. Goodeniaceae. – In: Steenis CGGJ
van (ed), Flora Malesiana I, 5(3), Noordhoff-Kolff N. V., Djakarta, pp.
335-344.
Leeson KE, Rozefelds AC. 2003. A new endemic
Ozothamnus species (Asteraceae) from Tasmania, Australia.
– Aust. Syst. Bot. 16: 317-322.
Leins P. 1968. Versuch einer Gliederung der
Inulinae und Buphthalminae nach den Pollenkorntypen. – Ber. Deutsch. Bot.
Ges. 81: 498-504.
Leins P. 1970. Die Pollenkörner und
Verwandtschaftsbeziehungen der Gattung Eremothamnus (Asteraceae). – Mitt. Bot.
Staatssamml. München 7: 369-376.
Leins P. 1971. Pollensystematische Studien an
Inuleen I. Tarchonanthinae, Plucheinae, Inulinae, Buphthalminae. – Bot.
Jahrb. Syst. 91: 91-146.
Leins P. 1973. Pollensystematische Studien an
Inuleen II. Filagininae. – Bot. Jahrb. Syst. 93: 603-611.
Leins P, Erbar C. 1987. Studien zur
Blütenentwicklung an Compositen. – Bot. Jahrb. Syst. 108: 381-401.
Leins P, Erbar C. 1989. Zur
Blütenentwicklung und sekundären Pollenpräsentation bei Selliera
radicans Cav. (Goodeniaceae). – Flora 182:
43-56.
Leins P, Erbar C. 1990. On the mechanisms of
secondary pollen presentation in the Campanulales-Asterales complex. –
Bot. Acta 103: 87-92.
Leins P, Erbar C. 2000. Die frühesten
Entwicklungsstadien der Blüten bei den Asteraceae. – Bot. Jahrb. Syst.
122: 503-515.
Leins P, Erbar C. 2003. The pollen box in
Cyphia (Cyphiaceae). – Intern. J. Plant Sci. 164(Suppl.):
S321-S328.
Leins P, Erbar C. 2005. Floral morphological
studies in the South African Cyphia stenopetala Diels (Cyphiaceae).
– Intern. J. Plant Sci. 166: 207-217.
Leins P, Erbar C. 2006. Secondary pollen
presentation syndromes of the Asterales – a phylogenetic perspective. –
Bot. Jahrb. Syst. 127: 83-103.
Leins P, Thyret G. 1971. Pollen phylogeny and
taxonomy exemplified by an African Asteraceae group. – Mitt. Bot.
Staatssamml. München 10: 280-286.
Leonhardt R. 1949.
Phylogenetisch-systematische Betrachtungen I. Betrachtungen zur Systematik der
Compositen. – Österr. Bot. Zeitschr. 96: 293-324.
Leonova T. 1987. Konspekt roda
Artemisia L. (Asteraceae)
flory Evropeiskoj chasti SSSR. – Nov. Sist. Vysš. Rast. 24: 177-201.
Lersten NR, Curtis JD. 1985. Distribution and
anatomy of hydathodes in Asteraceae. – Bot. Gaz. 146:
106-114.
Lersten NR, Curtis JD. 1987. Internal
secretory spaces in Asteraceae. A
review and original observations on Conyza canadensis (tribe
Astereae). – La Cellule 74: 181-196.
Lersten NR, Curtis JD. 1989. Polyacetylene
reservoir (duct) development in Ambrosia trifida (Asteraceae) staminate flowers –
Amer. J. Bot. 76: 1000-1005.
Leyens T, Lobin W. 1995. Campanula
(Campanulaceae) on the Cape
Verde Islands – two species or only one? – Willdenowia 25: 215-228.
Li S-P, Hsieh T-H, Lin C-C. 2002. The genus
Nymphoides Séguier (Menyanthaceae) in Taiwan. –
Taiwania 47: 246-258.
Li W-P, Yang F-S, Jivkova T, Yin G-S. 2012.
Phylogenetic relationships and generic delimitation of Eurasian Aster
(Asteraceae: Astereae) inferred
from ITS, ETS and trnL-F sequence data. – Ann. Bot. 109:
1341-1357.
Li W-P, Qian F-M, Yang X-L, Chen S-M. 2014.
Systematic position of Cyathocline Cass. (Asteraceae): evidences from
molecular, cytological and morphological data. – Plant Syst. Evol. 300:
595-606.
Linder C, Goertzen L, Vanden Heuvel B,
Francisco-Ortega J, Jansen RK. 2000. The complete external transcribed spacer
of 18S-26S rDNA: amplification and phylogenetic utility at low taxonomic levels
in Asteraceae and closely allied
families. – Mol. Phylogen. Evol. 14: 285-303.
Lindsey AA. 1938. Anatomical evidence for the
Menyanthaceae. – Amer. J.
Bot. 25: 480-485.
Ling T, Zhang Z, Xia T, Ling W, Wan X. 2009.
Phytoecdysteroids and other constituents from the roots of Klaseopsis
chinensis. – Biochem. Syst. Ecol. 37: 49-51.
Ling Y, Chen Y-L. 1965. Genera nova del minus
cognita familiae Compositarum II. Cavea W. W. Smith et Small et
Nannoglottis Maxim. – Acta Phytotaxon. Sin. 10: 91-104.
Ling Y, Chen Y-L. 1973. Notulae de genere
Erigeron L. generibusque affinibus florae Sinicae. – Acta
Phytotaxon. Sin. 11: 399-430.
Ling Y, Tseng YQ. 1978. Miscellaneous notes
on Chinese Compositae. – Acta Phytotaxon. Sin. 16: 82-86.
Ling Y-R. 1992. The Old World
Artemisia Linn. (Compositae). – Bull. Bot. Res. Harbin I, 12:
1-108.
Lippert W. 1973. Revision der Gattung
Aster in Afrika. – Mitt. Bot. Staatssamml. München 11: 153-258.
Lipschitz SJ. 1979. Rod Saussurea
DC. – Leningrad.
Lipšić SJ. 1935. Fragmenta monographiae
generis Scorzonera l. – Moscow.
Lipšić SJ. 1954. De genere novo
Frolovia (DC.) Lipsch. – Bot. Mater. Gerb. Bot. Inst. Komarova Akad.
Nauk SSSR 16: 461-462. [In Russian]
Lipšić SJ. 1961. Ad cognitionem generis
Saussurea DC. florae URSS. – Bot. Mater. Gerb. Bot. Inst. Komarova
Akad. Nauk SSSR 21: 369-381. [In Russian]
Lipšić SJ. 1979. Rod Saussurea DC.
– Leningrad.
Lisowski S. 1991. Les Asteraceae dans la flore d’Afrique
central (excl. Cichorieae, Inuleae et Vernonieae) 2. – Fragm. Flor. Geobot.
36 (Suppl. 1): 251-627.
Liston A, Kadereit JW. 1995. Chloroplast DNA
evidence for introgression and long distance dispersal in the desert annual
Senecio flavus (Asteraceae). – Plant Syst. Evol.
197: 33-41.
Liston A, Rieseberg LH, Elias TS. 1989.
Genetic similarity is high between intercontinental disjunct species of
Senecio. – Amer. J. Bot. 76: 383-388.
Liu H, Trusty J, Oviedo R, Anderberg AA,
Francisco-Ortega J. 2004. Molecular phylogenetics of the Caribbean genera
Rhodogeron and Sachsia (Asteraceae). – Intern. J. Plant
Sci. 165: 209-217.
Liu J-Q. 1999. Systematics of the tribe
Senecioneae Subtribe Tussilagininae (Asteraceae) of the Eastern Asia. –
Ph.D. diss., Institute of Botany, Chinese Academy of Sciences, Beijing.
Liu J-Q. 2000. Pollen wall ultrastructures of
subtribe Tussilagininae (Asteraceae: Senecioneae) of the
Eastern Asia and their systematic and taxonomic significance. – J. Wuhan Bot.
Res. 18: 461-465.
Liu J-Q. 2001. Floral microcharacters of the
subtribe Tussilagininae (Asteraceae: Senecioneae) of the
Eastern Asia and their systematic and taxonomic significance. – Bull. Bot.
Res. 21: 11-27.
Liu J-Q. 2004. Uniformity of karyotypes in
Ligularia (Asteraceae:
Senecioneae), a highly diversified genus of the eastern Qinghai-Tibet Plateau
highlands and adjacent areas. – Bot. J. Linn. Soc. 144: 329-342.
Liu J-Q, Liu S-W, Ho T-N, Lu A-M. 2001.
Karyological studies on the Sino-Himalayan genus, Cremanthodium (Asteraceae: Senecioneae). – Bot. J.
Linn. Soc. 135: 107-112.
Liu J-Q, Gao T-G, Chen Z-D, Lu A-M. 2002.
Molecular phylogeny and biogeography of the Qinghai-Tibet Plateau endemic
Nannoglottis (Asteraceae). – Mol. Phylogen. Evol.
23: 307-325.
Liu J-Q, He Y-P, Kong H-Z. 2002. The pollen
characteristics under SEM in Farfugium and Ligulariopsis and
their taxonomic significance. – Acta Bot. Boreal.-Occid. Sin. 22: 33-36.
Liu J-Q, Wang Y-J, Wang A-L, Hideaki O,
Abbott RJ. 2006. Radiation and diversification within the
Ligularia-Cremanthodium-Parasenecio complex (Asteraceae) triggered by uplift of
the Qinghai-Tibetan Plateau. – Mol. Phylogen. Evol. 38: 31-49.
Liu M, Zhang CF, Huang CH, Ma H. 2015.
Phylogenetic reconstruction of tribal relationships in Asteroideae (Asteraceae) with low-copy nuclear
genes. – Chin. Bull. Bot. 50: 549-564.
Liu S-W, Ho T-N, Chen S-L, Liu J-Q. 2002.
Origin and distribution of the genus Cremanthodium Benth. – Acta
Biol. Plateau Sin. 15: 53-61.
Liu Y, Yang Q-E. 2011. Hainanecio, a
new genus of the Senecioneae, Asteraceae from China. – Bot. Stud.
52: 115-120.
Liu Y, Deng T, Yang Q-E. 2012. Karyology of
the genus Faberia (Cichorieae-Asteraceae) and its systematic
implications. – Nord. J. Bot. 30: 365-371.
Liveri E, Bareka P, Kamari G. 2018. Taxonomic
study on the Greek endemic genus Hymenonema (Asteraceae:
Cichorieae), using morphological and karyological traits. –
Willdenowia 48: 5-21.
Lloyd DG, Webb CJ. 1987. The reinstatement of
Leptinella at generic rank, and the status of the “Cotuleae” (Asteraceae, Anthemideae). – New
Zealand J. Bot. 25: 99-105.
Lobova TA. 1997. Seed morphology and anatomy
in the genera Argophyllum and Corokia (Argophyllaceae). – Bot. Žurn.
82: 68-78. [In Russian]
Lobreau-Callen D. 1977. Famille des Phellinaceae. Les pollens des
Célastrales. – École Pratique des Hautes Études, Institut de Montpellier,
pp. 42-43.
Loesener T. 1901. Monographia Aquifoliacearum
I. – Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 78: 504-516.
Loesener T. 1942. Aquifoliaceae. –
In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen
Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 36-86.
Loeuille B, Deble L, Nakajima JN. 2011. Four
new species of Chionolaena (Asteraceae: Gnaphalieae) from
south-eastern Brazil. – Kew Bull. 66: 263-272.
Loeuille B, Nakajima JN, Oliveira DMT, Semir
J, Pirani JR. 2013. Two new species of Heterocoma (Asteraceae: Vernonieae) and a
broadened concept of the genus. – Syst. Bot. 38: 242-252.
Loeuille B, Keeley SC, Pirani JR. 2015.
Systematics and evolution of syncephaly in American Vernonieae (Asteraceae) with emphasis on the
Brazilian subtribe Lychnophorinae. – Syst. Bot. 40: 286-298.
Loeuille B, Semir J, Lohmann LG, Pirani JR.
2015. A phylogenetic analysis of Lychnophorinae (Asteraceae: Vernonieae) based on
molecular and morphological data. – Syst. Bot. 40: 299-315.
Lohwasser U, Granda A, Blattner FR. 2004.
Phylogenetic analysis of Microseris (Asteraceae), including a newly
discovered Andean population from Peru. – Syst. Bot. 29: 774-780.
Longpre EK. 1970. The systematics of the
genera Sabazia, Selloa, and Tricarpha (Compositae).
– Publ. Mus. Michigan State Univ. 4: 287-383.
Loockerman DJ. 1996. Phylogenetic and
molecular evolutionary studies of the Tageteae (Asteraceae). – Ph.D. diss.,
University of Texas, Austin, Texas.
Loockerman DJ, Turner BL, Jansen RK. 2003.
Phylogenetic relationships within the Tageteae (Asteraceae) based on nuclear
ribosomal ITS and chloroplast ndhF gene sequences. – Syst. Bot. 28:
191-207.
López MG, Wulff AF, Poggio L, Xifreda CC.
2005. Chromosome numbers and meiotic studies in species of Senecio (Asteraceae) from Argentina. – Bot.
J. Linn. Soc. 148: 465-474.
López González G. 2012. Sobre la
clasificación del complejo Carthamus-Carduncellus (Asteraceae, Cardueae-Centaureinae) y
su tratamiento en Flora Iberica. – Acta Bot. Malacitana 37: 79-92.
López-Sepúlveda P, Tremetsberger K, Ortiz
MÁ, Baeza CM, Peñailillo P, Stuessy TF. 2013. Radiation of the
Hypochaeris apargioides complex (Asteraceae: Cichorieae) of southern
South America. – Taxon 62: 550-564.
López-Vinyallonga S, Mehregan I,
Garcia-Jacas N, Tscherneva O, Susanna A, Kadereit JW. 2009. Phylogeny and
evolution of the Arctium-Cousinia complex (Compositae,
Cardueae-Carduinae). – Taxon 58: 153-171.
López-Vinyallonga S, Romaschenko K, Susanna
A, Garcia-Jacas N. 2011. Systematics of the arctioid group: disentangling
Arctium and Cousinia (Cardueae, Carduinae). – Taxon 60:
539-554.
Lo Presti RM, Oberprieler C. 2009.
Evolutionary history, biogeography and eco-climatological differentiation of
the genus Anthemis L. (Compositae, Anthemideae) in the
circum-Mediterranean area. – J. Biogeogr. 136: 1313-1332.
Lo Presti RM, Oppolzer S, Oberprieler C.
2010. A molecular phylogeny and a revised classification of the Mediterranean
genus Anthemis s.l. (Compositae, Anthemideae) based on three molecular
markers and micromorphological characters. – Taxon 59: 1441-1456.
Löve D, Dansereau P. 1959. Biosystematic
studies on Xanthium: taxonomic appraisal and ecological status. –
Can. J. Bot. 37: 173-208.
Lowrey TK. 1986. A biosystematic revision of
Hawaiian Tetramolopium (Compositae-Astereae). – Allertonia 4:
203-265.
Lowrey TK. 1995. Phylogeny, adaptive
radiation, and biogeography of Hawaiian Tetramolopium (Asteraceae, Astereae). – In: Wagner
WL, Funk VA (eds), Hawaiian biogeography: evolution on a hot spot archipelago,
Smithsonian Institution Press, Washington, D.C., pp. 195-220.
Lowrey TK, Crawford DJ. 1985. Allozyme
divergence and evolution in Tetramolopium (Compositae-Astereae) on the
Hawaiian Islands. – Syst. Bot. 10: 64-72.
Lowrey TK, Urbatsch L. 2003. Phylogenetic
studies in the Astereae: the need for generic redelimitation and a reasonable
subtribal classification. – Compositae Newslett. 40: 25.
Lowrey TK, Quinn CJ, Taylor RK, Chan R,
Kimball RT, Nrdi JC de. 2001. Molecular and morphological reassessment of
relationships within the Vittadinia group of Astereae (Asteraceae). – Amer. J. Bot. 88:
1279-1289.
Lowrey TK, Whitkus R, Sykes WR. 2005. A new
species of Tetramolopium (Asteraceae) from Mitiaro, Cook
Islands: biogeography, phylogenetic relationships, and dispersal. – Syst.
Bot. 30: 448-455.
Lowrie A, Carlquist SJ. 1991. Studies in
Stylidium from Western Australia: new taxa, rediscoveries and range
extensions. – Phytologia 71: 5-28.
Lowrie A, Kenneally KF. 1994. Stylidium
pulviniforme (Stylidiaceae), a new species of
triggerplant from south-west Western Australia. – Nuytsia 9: 369-373.
Lowrie A, Kenneally KF. 1996. Stylidium
fimbriatum (Stylidiaceae),
a new tropical species of triggerplant from the Kimberley, Western Australia.
– Nuytsia 10: 425-427.
Lowrie A, Kenneally KF. 1997a. Six new
species of triggerplant (Stylidium: Stylidiaceae) from south-west
Western Australia. – Nuytsia 11: 185-198.
Lowrie A, Kenneally KF. 1997b. Eight new
species of triggerplants (Stylidium: Stylidiaceae) from northern
Australia. – Nuytsia 11: 199-217.
Lowrie A, Kenneally KF. 1997c. A taxonomic
review of Stylidium subgenus Forsteropsis (Stylidiaceae). – Nuytsia 11:
353-364.
Lowrie A, Kenneally KF. 1998a. Three new
species of triggerplant (Stylidium: Stylidiaceae) from south-west
Western Australia. – Nuytsia 12: 75-82.
Lowrie A, Kenneally KF. 1998b. Three new
triggerplant species in Stylidium subgenus Centridium (Stylidiaceae) from Western
Australia. – Nuytsia 12: 197-206.
Lowrie A, Kenneally KF. 1999. Stylidium
candelabrum (Stylidiaceae),
a new species from the Northern Territory, Australia. – Nuytsia 13:
251-254.
Lowrie A, Kenneally KF. 2000. Three new
species of Stylidium (Stylidiaceae) from south-west
Western Australia. – Nuytsia 13: 293-302.
Lowrie A, Coates DJ, Kenneally KF. 1998. A
taxonomic review of the Stylidium caricifolium complex (Stylidiaceae), from south-west
Western Australia. – Nuytsia 12: 43-57.
Lowrie A, Burbidge AH, Kenneally KF. 1999. A
taxonomic revision of the creeping triggerplants (Stylidiaceae: Stylidium
sect. Appressae) from southern Australia. – Nuytsia 13: 89-157.
Lowrie A, Coates DJ, Kenneally KF. 1999.
Stylidium chiddarcoopingense (Stylidiaceae), a new species from
south-west Western Australia. – Nuytsia 13: 255-257.
Lu S-T, Akan H, Civelek E. 2003. A new
species of Centaurea (Asteraceae: sect.
Psephelloideae) from Turkey. – Bot. J. Linn. Soc. 143: 207-212.
Ludwig M. 2011. The molecular evolution of
β-carbonic anhydrase in Flaveria. – J. Experim. Bot. 62:
3071-3081.
Luebert F, Wen J, Dillon MO. 2009. Systematic
placement and biogeographical relationships of the monotypic genera
Gypothamnium and Oxyphyllum (Asteraceae: Mutisioideae) from the
Atacama Desert. – Bot. J. Linn. Soc. 159: 32-51.
Luebert F, Moreira-Muñoz A, Wilke K, Dillon
MO. 2017. Phylogeny and evolution of achenial trichomes in the
Lucilia-group (Asteraceae: Gnaphalieae) and their
systematic significance. – Taxon 66: 1184-1199.
Lundberg J. 2001a. The asteralean affinity of
the Mauritian Roussea (Rousseaceae). – Bot. J. Linn. Soc.
137: 267-276.
Lundberg J. 2001b. Phylogenetic studies in
the euasterids II: with particular reference of Asterales and Escalloniaceae.
– Online article at website:
http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-1597
Lundberg J. 2009. Asteraceae and relationships within
Asterales. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics,
evolution, and biogeography of Compositae, International Association for Plant
Taxonomists, Wien, pp. 157-169.
Lundberg J, Bremer K. 2003. A phylogenetic
study of the order Asterales using one morphological and three molecular data
sets. – Intern. J. Plant Sci. 164: 553-578.
Lundgren J. 1972. Revision of the genus
Anaxeton Gaertn. (Compositae). – Opera Bot. 31: 1-59.
Lundgren J. 1974. The genus
Petalacte D. Don (Compositae). – Bot. Not. 127: 119-124.
Lundin R. 2006. Nordenstamia Lundin
(Compositae-Senecioneae), a new genus from the Andes of South America. –
Compositae Newslett. 44: 14-18.
Lusa MG, Martucci MEP, Loeuille BFP,
Gobbo-Neto L, Appezzato-da-Glória B, Da Costa FB. 2016. Characterization and
evolution of secondary metabolites in Brazilian Vernonieae (Asteraceae) assessed by LC-MS
fingerprinting. – Bot. J. Linn. Soc. 182: 594-611.
Luteyn JL. 1986. A new Burmeistera
(Campanulaceae: Lobelioideae)
from western Colombia. – Syst. Bot. 11: 474-476.
Mabberley DJ. 1974a. The pachycaul lobelias
of Africa and St. Helena. – Kew Bull. 29: 535-589.
Mabberley DJ. 1974b. Pachycauly,
vessel-elements, islands and the evolution of arborescence in ‘herbaceous’
families. – New Phytol. 73: 977-984.
Mabberley DJ. 1975. The giant lobelias:
pachycauly, biogeography, ornithophily and continental drift. – New Phytol.
74: 365-374.
Mabry TJ, Bohlmann F. 1977. Summary of the
chemistry of the Compositae. – In: Heywood VH, Harborne JB, Turner BL (eds),
The biology and chemistry of the Compositae, Academic Press, London, pp.
1097-1104.
Mabry TJ, Wagenitz G (eds). 1990. Research
advances in the Compositae. – Plant Syst. Evol. [Suppl.] 4: 1-124.
Mabry TJ, Timmermann BN, Heil N, Power AM.
1981. Systematic implications of the flavonoids and chromosomes of
Flyriella (Compositae-Eupatorieae). – Plant Syst. Evol. 137:
275-280.
McGregor RL. 1968. The taxonomy of the genus
Echinacea (Compositae). – Univ. Kansas Sci. Bull. 48: 113-142.
McKenzie RJ, Barker NP. 2008. Radiation of
southern African daisies: biogeographic inferences for subtribe Arctotidinae
(Asteraceae, Arctotideae). –
Mol. Phylogen. Evol. 49: 1-16.
McKenzie RJ, Ward JM, Lovis JD, Breitwieser
I. 2004. Morphological evidence for natural intergeneric hybridization in the
New Zealand Gnaphalieae (Compositae): Anaphalioides bellidioides x
Ewartia sinclairii. – Bot. J. Linn. Soc. 145: 59-75.
McKenzie RJ, Samuel J, Muller EM, Skinner
AKW, Barker NP. 2005. Morphology of cypselae in subtribe Arctotidinae
(Compositae-Arctotideae) and its taxonomic implications. – Ann. Missouri Bot.
Gard. 92: 569-594.
McKenzie RJ, Mitchell SD, Barker NP. 2006. A
new species of Arctotis (Compositae, Arctotideae) from kommetjie
grassland in Eastern Cape Province, South Africa. – Bot. J. Linn. Soc. 151:
581-588.
McKown AD, Dengler NG. 2009. Shifts in leaf
vein density through accelereated vein formation in C4
Flaveria (Asteraceae).
– Ann. Bot. 104: 1085-1098.
McKown AD, Moncalvo JM, Dengler NG. 2005.
Phylogeny of Flaveria (Asteraceae) and inference of
C4 photosynthesis evolution. – Amer. J. Bot. 92: 1911-1928.
MacLeish NFF. 1984. Eight new combinations in
Vernonia (Compositae: Vernonieae). – Syst. Bot. 9: 133-136.
MacLeish NFF. 1985a. Revision of
Glaziovianthus (Compositae: Vernonieae). – Syst. Bot. 10:
347-352.
MacLeish NFF. 1985b. Revision of
Chresta and Pycnocephalum (Compositae: Vernonieae). – Syst.
Bot. 10: 459-470.
MacLeish NFF, Schumacher H. 1984. Six new
species of Eremanthus (Compositae: Vernonieae) from Brazil. – Syst.
Bot. 9: 84-94.
McVaugh R. 1940. A revision of
“Laurentia” an allied genera in North America. – Bull. Torrey
Bot. Club 67: 778-798.
McVaugh R. 1941a. A monograph of the genus
Downingia. – Mem. Torrey Bot. Club 19: 1-57.
McVaugh R. 1941b. A new name for
Heterocodon rariflorum Nutt. – Leafl. W. Bot. 3: 48.
McVaugh R. 1945. The genus Triodanis
Rafinesque, and its relationship to Specularia and Campanula.
– Wrightia 1: 13-52.
McVaugh R. 1948. Generic status of
Triodanis and Specularia. – Rhodora 50: 38-49.
McVaugh R. 1949. Studies in South American
Lobelioideae (Campanulaceae)
with special reference to Colombian species. – Brittonia 6: 450-493.
Magee AR, Tilney PM. 2012. A taxonomic
revision of Pentzia (Asteraceae, Anthemideae) I: the
P. incana group in southern Africa, including the description of the
new species P. oppositifolia Magee. – South Afr. J. Bot. 79:
148-158.
Magee AR, Nicolas AN, Tilney PM, Plunkett GM.
2015. Phylogenetic relationships and generic realignments in the early
diverging subtribe Pentziinae (Asteraceae, Anthemideae). – Bot. J.
Linn. Soc. 178: 633-647.
Magee AR, Ebrahim I, Koopman R, Staden L von.
2017. Marasmodes (Asteraceae, Anthemideae), the most
threatened plant genus of the Cape Floristic Region, South Africa: conservation
and taxonomy. – South Afr. J. Bot. 111: 371-386.
Magoswana SL, Magee AR. 2014. A taxonomic
revision of Hymenolepis (Asteraceae, Anthemideae). – South
Afr. J. Bot. 91: 126-141.
Magoswana SL, Boatwright JS, Manning JC,
Magee AR. 2016. A taxonomic revision of Inulanthera (Asteraceae: Anthemideae). – South
Afr. J. Bot. 105: 141-157.
Magoswana SL, Boatwright JS, Magee AR,
Manning JC. 2016. A taxonomic revision of Gymnodiscus (Asteraceae: Senecioneae: Othonninae),
a Greater Cape Floristic Region endemic. – South Afr. J. Bot. 106: 71-77.
Maguire B. 1943. A monograph of the genus
Arnica (Senecioneae, Compositae). – Brittonia 4: 386-510.
Maguire B. 1956. Distribution, endemicity,
and evolution patterns among Compositae of the Guyana Highland of Venezuela.
– Proc. Amer. Philos. Soc. 100: 467-475.
Maguire B. 1967. Compositae. – In: Maguire
B (ed), The botany of the Guayana Highland VII, Mem. New York Bot. Gard. 17:
437-439.
Maguire B, Wurdack JJ. 1957. The botany of
the Guayana Highland II. – Mem. New York Bot. Gard. 9: 235-392.
Maguire B, Steyermark JA, Wurdack JJ. 1957.
Botany of the Chimanta Massif I. Gran Sabana, Venezuela, Mem. New York Bot.
Gard. 9: 393-439.
Maheshwari P. 1944. The origin of the
haustoria in the ovule of Lobelia. – Curr. Sci. 13: 186-187.
Maheshwari P, Srinivasan AR. 1944. A
contribution to the embryology of Rudbeckia bicolor Nutt. – New
Phytol. 43: 135-142.
Mai DH. 2000. Lobelia pliocenica
(Dorofeev) comb. nova – ein neues atlantisches Florenelement im Jungtertiär
und Altquartär Europas. – Feddes Repert. 111: 481-492.
Maier A, Emig W, Leins P. 1999. Dispersal
patterns of some Phyteuma species (Campanulaceae). – Plant Biol. 1:
408-417.
Małecka J. 1962. Cytological studies in the
genus Taraxacum. – Acta Biol. Cracov., Ser. Bot., 5: 117-136.
Małecka J. 1970. Cyto-taxonomic studies in
the genus Taraxacum section Palustria Dahlstedt. – Acta
Biol. Cracov., Ser. Bot., 13: 155-168.
Małecka J. 1972. Further cyto-taxonomic
studies in the genus Taraxacum section Palustria Dahlstedt.
– Acta Biol. Cracov., Ser. Bot., 15: 113-126.
Małecka J. 1982. Further embryological
studies in the genus Taraxacum L. – Acta Biol. Cracov., Ser. Bot.,
24: 143-157.
Malme GOA. 1899. Die Compositen der Ersten
Regnellschen Expedition. – Öfvers. Kongl. Vetensk.-Akad. Förh., Ser. 2, 32:
1-90.
Malme GOA. 1933. Compositae Paranenses
Dusenianae. – Kongl. Vetensk.-Akad. Handl., Ser. 3, 12: 1-122.
Mandel JR, Dikow RB, Funk VA, Masalia RR,
Staton SE, Kozik A, Michelmore RW, Rieseberg LH, Burke JM. 2014. A target
enrichment method for gathering phylogenetic information from hundreds of loci:
an example from the Compositae. – Appl. Plant Sci. 2: apps. 1300085. doi:
10.3732/apps.1300085
Mandel JR, Dikow RB, Funk VA. 2015. Using
phylogenomics to resolve mega-families: an example from Compositae. – J.
Syst. Evol. 53: 391-402.
Mandel JR, Barker MS, Bayer RJ, Dikow RB, Gao
T-G, Jones KE, Keeley S, Kilian N, Ma H, Siniscalchi CM, Susanna A, Thapa R,
Watson L, Funk VA. 2017. The Compositae tree of life in the age of
phylogenomics. – J. Syst. Evol. 55: 405-410.
Manilal KS. 1971. Vascularization of the
corolla of the Compositae. – J. Indian Bot. Soc. 50: 189-196.
Manning JC, Simka B, Boatwright JS, Magee AR.
2016. A revised taxonomy of Gerbera sect. Gerbera (Asteraceae: Mutisieae). – South
Afr. J. Bot. 104: 142-157.
Mansanares ME, Forni-Martins ER, Semir J.
2007. Cytotaxonomy of Lychnophoriopsis Sch. Bip. and
Paralychnophora MacLeish species (Asteraceae: Vernonieae:
Lychnophorinae). – Bot. J. Linn. Soc. 154: 109-114.
Maraner F, Samuel R, Stuessy T, Crawford D,
Crisci J, Pandey A, Mort M. 2012. Molecular phylogeny of Nassauvia (Asteraceae, Mutisieae) based on nrDNA
ITS sequences. – Plant Syst. Evol. 298: 399-408.
Markgraf F. 1950. Die Campanulaceen von
Südwestafrika. – Bot. Jahrb. Syst. 75: 206-220.
Markos S, Baldwin BG. 2001. Higher-level
relationships and major lineages of Lessingia (Compositae, Astereae)
based on nuclear rDNA internal and external transcribed spacer (ITS and ETS)
sequences. – Syst. Bot. 26: 168-183.
Markos S, Baldwin BG. 2002. Structure,
molecular evolution, and phylogenetic utility of the 5’ region of the
external transcribed spacer of 18S-26S rDNA in Lessingia (Compositae,
Asteraceae). – Mol. Phylogen.
Evol. 23: 214-228.
Marlowe K, Hufford L. 2007. Taxonomy and
biogeography of Gaillardia (Asteraceae): a phylogenetic analysis.
– Syst. Bot. 32: 208-226.
Marticorena C, Parra O. 1974. Morfología de
los granos de polen y posición sistemática de Anisochaeta DC.,
Chionopappus Benth., Feddea Urb. y Gochnatia
glomeriflora Gray (Compositae). – Bol. Soc. Biol. Concepción 47:
187-197.
Marticorena C, Parra O. 1975. Morfología de
los granos de pollen de Hesperomannia Gray y Moquinia DC.
(Compositae-Astereae). Estudio comparativo con generos afines. – Gayana, Bot.
29: 3-22.
Martín J, Torrell M, Korobkov AA, Vallés J.
2003. Palynological features as a systematic marker in Artemisia L.
and related genera (Asteraceae,
Anthemideae) II. Implications for subtribe Artemisiinae delimitation. – Plant
biol. 5: 85-93.
Martin PG, Peacock WJ. 1959. Pollen tetrad
patterns in Leschenaultia. – Proc. Linn. Soc. New South Wales 87:
388-396.
Martinoli G. 1953. Studio citotassonomico dei
generi Hyoseris e Robertia con particulare referimento all’
Hyoseris taurina G. Martinoli sp. nov. (Asteraceae). – Caryologia 5:
253-281.
Martins L. 2006a. Systematics and
biogeography of Klasea (Asteraceae-Cardueae) and a synopsis
of the genus. – Bot. J. Linn. Soc. 152: 435-464.
Martins L. 2006b. Klaseopsis and
Archiserratula – two new genera segregated from Serratula
(Compositae, Cardueae). – Taxon 55: 973-976.
Martins L, Hellwig FH. 2005a. Phylogenetic
relationships of the enigmatic species Serratula chinensis and
Serratula forrestii (Asteraceae-Cardueae). – Plant Syst.
Evol. 255: 215-224.
Martins L, Hellwig FH. 2005b. Systematic
position of the genera Serratula and Klasea within
Centaureinae (Cardueae, Asteraceae) inferred from ETS and ITS
sequence data and new combinations in Klasea. – Taxon 54:
632-638.
Mason CM. 2018. How old are sunflowers? A
molecular clock analysis of key divergences in the origin and diversification
of Helianthus (Asteraceae). – Intern. J. Plant
Sci. 179: 182-191.
Mathew A, Mathew PM. 1976. Studies on south
Indian Compositae II. Cytology of the genus Vernonia Schreb. –
Cytologia 41: 401-406.
Mathew A, Mathew PM. 1988. Cytological
studies on the South Indian Compositae. – Glimpses Plant Res. 8: 1-177.
Mathew PM, Mathew A. 1983. Studies on the
South Indian Compositae V. Cytotaxonomic considerations of the tribe Vernonieae
and Eupatorieae. – Cytologia 48: 679-690.
Mattfeld J. 1934. VII. Compositae novae
sinenses. – Notizbl. Bot. Gart. Mus. Berlin-Dahlem 11: 102-110.
Mattfeld J. 1940. Einige neuen Compositen aus
dem Gebiet des Saruwaged-Gebirges in Neuginea. – Bot. Jahrb. Syst. 70:
470-482.
Mauritzon J. 1933. Studien über Embryologie
der Familien Crassulaceae und
Saxifragaceae.
– Ph.D. diss, University of Lund, Sweden.
Mavrodiev EV, Edwards CE, Albach DC,
Gitzendanner MA, Soltis PS, Soltis DE. 2004. Phylogenetic relationships in
subtribe Scorzonerinae (Asteraceae: Cichorioideae:
Cichorieae) based on ITS sequence data. – Taxon 53: 699-712.
Mavrodiev EV, Tancig M, Sherwood AM,
Gitzendanner MA, Rocca J, Soltis PS, Soltis DE. 2005. Phylogeny of
Tragopogon L. (Asteraceae) based on internal and
external transcribed spacer sequence data. – Intern. J. Plant Sci. 166:
117-133.
Mavrodiev EV, Soltis PS, Soltis DE. 2008.
Putative parentage of six Old World polyploids in Tragopogon L. (Asteraceae: Scorzonerinae) based on
ITS, ETS, and plastid sequence data. – Taxon 57: 1215-1232.
Mehra PN, Remanandan P. 1974. Cytological
investigations on the Indian Compositae II. Astereae, Heliantheae, Helenieae,
and Anthemideae. – Caryologia 27: 255-284.
Mehra PN, Gill BS, Mehta JK, Sidhu SS. 1965.
Cytological investigations on the Indian Compositae I. North-Indian taxa. –
Caryologia 18: 35-68.
Mehregan I. 2009. Identity, relationship and
distribution of the poorly known Cousinia elata (Asteraceae, Cardueae). –
Willdenowia 39: 83-87.
Mehregan I, Kadereit JW. 2008. Taxonomic
revision of Cousinia sect. Cynaroideae (Asteraceae, Cynareae). –
Willdenowia 38: 293-362.
Mehregan I, Kadereit JW. 2009. The role of
hybridization in the evolution of Cousinia s. str. (Asteraceae, Cardueae). –
Willdenowia 39: 35-47.
Meiri L, Dulberger R. 1986. Stamen filament
structure in the Asteraceae: the
anther collar. – New Phytol. 104: 693-701.
Mejías JA. 1993a. Cytotaxonomic studies in
the Iberian taxa of the genus Lactuca (Compositae). – Bot. Helvetica
103: 113-130.
Mejías JA. 1993b. Estudio cariológico del
género Launaea Cass. en la Península Iberica. – Lagascalia 17:
135-149.
Melo-de-Pinna GFA, Menezes NL. 2002.
Vegetative organ anatomy of Ianthopappus corymbosus Roque & Hind
(Asteraceae-Mutisieae). – Rev.
Brasileira Bot. 25: 505-514.
Merrett MF, Clarkson BD. 2000. Reinstatement
of Alseuosmia quercifolia (Alseuosmiaceae) from New Zealand.
– New Zealand J. Bot. 38: 153-164.
Merxmüller H. 1950. Compositen-Studien I.
– Mitt. Bot. Staatssamml. München 2: 33-46.
Merxmüller H. 1953. Compositen-Studien III.
Revision der Gattung Geigeria Griesslich. – Mitt. Bot. Staatssamml.
München 5: 239-316.
Merxmüller H. 1954. Compositen-Studien IV.
Die Compositen-Gattungen Südwestafrikas. Über einige mit Aster nahe
verwandten Gattungen Südwestafrikas. – Mitt. Bot. Staatssamml. München 1:
417-421.
Merxmüller H. 1965. Compositen-Studien VII.
Othonna in Südwestafrika. – Mitt. Bot. Staatssamml. München 17:
627-643.
Merxmüller H. 1966. Die sukkulenten
Senecionen Südwestafrikas. – Bot. Not. 119: 121-135.
Merxmüller H, Grau J. 1977.
Fruchtanatomische Untersuchungen in der Inula-Gruppe (Asteraceae). – Publ. Cairo Univ.
Herb. 7-8: 9-20.
Merxmüller H, Leins P, Roessler H. 1977.
Inuleae –systematic review. – In: Heywood VH, Harborne JB, Turner BL (eds),
The biology and chemistryof the Compositae 1, Academic Press, London, pp.
577-602.
Messina A, Walsh NG, Hoebee SE, Green PT.
2013. A morphological assessment of the Olearia phlogopappa complex
(Asteraceae: Astereae). – Aust.
Syst. Bot. 26: 31-80.
Messina A, Walsh NG, Hoebee SE, Green PT.
2014. A revision of Olearia section Asterotriche (Asteraceae: Astereae). – Aust.
Syst. Bot. 27: 199-240.
Meusel H, Kästner A. 1990. Lebensgeschichte
der Gold- und Silberdisteln. Monographie der mediterran-mitteleuropäischen
Compositen-Gattung Carlina I. – Österr. Akad. Wiss.,
Math.-Naturwiss. Kl., Denkschr. 127.
Meusel H, Kästner A. 1994. Lebensgeschichte
der Gold- und Silberdisteln. Monographie der mediterran-mitteleuropäischen
Compositen-Gattung Carlina II. – Österr. Akad. Wiss.,
Math.-Naturwiss. Kl., Denkschr. 128.
Meusel H, Ohle H. 1966. Zur Taxonomie und
Cytologie der Gattung Calendula. – Österr. Bot. Zeitschr. 113:
191-210.
Meyberg M. 1988. Cytochemistry and
ultrastructure of the mucilage secretory trichomes of Nymphoides
peltata (Menyanthaceae).
– Ann. Bot., N. S., 62: 537-547.
Miao B, Turner BL, Mabry TJ. 1995. Systematic
implications of chloroplast DNA variation in the subtribe Ambrosiinae (Asteraceae: Heliantheae). – Amer.
J. Bot. 82: 924-932.
Miao B, Turner BL, Mabry TJ. 1995.
Chloroplast DNA variations in sect. Cyclachaena of Iva (Asteraceae). – Amer. J. Bot. 82:
919-923.
Miao B, Turner BL, Simpson B, Mabry TJ. 1995.
Chloroplast DNA study of the genera Ambrosia s.l. and
Hymenoclea (Asteraceae):
systematic implications. – Plant Syst. Evol. 194: 241-255.
Michaels HJ, Scott KM, Olmstead RG, Szaro T,
Jansen RK, Palmer JD. 1993. Interfamilial relationships of the Asteraceae – insights from
rbcL sequence variation. – Ann. Missouri Bot. Gard. 80: 742-751.
Michalska K, Stojakowska A, Malarz J,
Dolezalova I, Lebeda A, Kisiel W. 2009. Systematic implications of
sesquiterpene lactones in Lactuca species. – Biochem. Syst. Ecol.
37: 174-179.
Millspaugh CF, Sherff EE. 1919. Revision of
the North American species of Xanthium. – Field Mus. nat. Hist.
Publ. 204, Bot. Ser. 4(2), Chicago.
Milstead WL. 1964. A revision of the North
American species of Prenanthes. – Ph.D. diss., Purdue University.
Mishler BD, Albert VA, Chase MW, Karis PO,
Bremer K. 1996. Character state weighting for DNA restriction site data:
asymmetry, ancestors, and the Asteraceae. – Cladistics 12:
11-19.
Misra S. 1972. Floral morphology of the
family Compositae IV. Tribe Vernonieae – Vernonia anthelmintica. –
Bot. Mag. (Tokyo) 85: 187-199.
Mitra JN. 1974. A contribution to the
embryology of some Compositae. – J. Indian Bot. Soc. 26: 105-123.
Mitsui Y, Chen S-T, Zhou Z-K, Peng C-I, Deng
Y-F, Setoguchi H. 2008. Phylogeny and biogeography of the genus
Ainsliaea (Asteraceae) in
the Sino-Japanese region based on nuclear rDNA and plastid DNA sequence data.
– Ann. Bot. 101: 111-124.
Mitsuoka S, Ehrendorfer F. 1972. Cytogenetics
and evolution of Matricaria and related genera (Asteraceae-Anthemideae). – Österr.
Bot. Zeitschr. 120: 155-200; 49: 605-615.
Mizukami H, Shimizu R, Kohiyouma M, Kohda H,
Kawanishi F, Hiraoka N. 1998. Phylogenetic analysis of Atractylodes
plants based on chloroplast trnK sequence. – Biol. Pharm. Bull. 21:
474-478.
Mitsuoka S, Ehrendorfer F. 1972. Cytogenetics
and evolution of Matricaria and related genera (Asteraceae-Anthemideae). – Österr.
Bot. Zeitschr. 120: 155-200.
Moeliono B, Tyun P. 1960. Campanulaceae. – In: Steenis
CGGJ van (ed), Flora Malesiana, I, 6(1), Noordhoff-Kolff N. V., Djakarta, pp.
107-141.
Moeser W. 1910. Die afrikanischen Arten der
Gattung Helichrysum Adans. – Engl. Bot. Jahrb. Syst. 44: 239-345.
Mogie M, Richards AJ. 1983. Satellited
chromosomes, systematics and phylogeny in Taraxacum (Asteraceae). – Plant Syst. Evol.
141: 219-229.
Molfino JF. 1953. Una nueva especie del
género Aphyllocladus. – Bol. Soc. Argentina Bot. 5: 30-34.
Montes-Moreno N, Sáez L, Benidí C, Susanna
A, Garcia-Jacas N. 2010. Generic delineation, phylogeny and subtribal
affinities of Phagnalon and Aliella (Compositae, Gnaphalieae)
based on nuclear and chloroplast sequences. – Taxon 59: 1654-1670.
Montes-Moreno N, Garcia-Jacas N, Benedí C,
Sáez L. 2013. Evaluation of the taxonomic status of the genus Aliella
(Compositae, Gnaphalieae): a recircumscription of the genus Phagnalon.
– Phytotaxa 148: 1-31.
Moore AJ, Bohs L. 2007. Phylogeny of
Balsamorhiza and Wyethia (Asteraceae: Heliantheae) using ITS,
ETS, and trnK sequence data. – Syst. Bot. 32: 682-691.
Moore AJ, Bartoli A, Tortosa RD, Baldwin BG.
2012. Phylogeny, biogeography, and chromosome evolution of the amphitropical
genus Grindelia (Asteraceae) inferred from nuclear
ribosomal and chloroplast sequence data. – Taxon 61: 211-230.
Moore MJ, Francisco-Ortega J, Santos-Guerra
A, Jansen RK. 2002. Chloroplast DNA evidence for the roles of island
colonization and extinction in Tolpis (Asteraceae: Lactuceae). – Amer. J.
Bot. 89: 518-526.
Moore RJ, Frankton C. 1962. Cytotaxonomic
studies in the tribe Cynareae (Compositae). – Can. J. Bot. 40: 281-293.
Moore S. 1902. A contribution to the
Composite flora of Africa. – J. Linn. Soc., Bot. 35: 305-367.
Mooring JS. 1997. A new base chromosome
number and phylogeny for Eriophyllum (Asteraceae, Helenieae). – Madroño
44: 364-373.
Moran E, Funk VA. 2006. A revision of
Erato (Compositae: Liabeae). – Syst. Bot. 31: 597-609.
Morefield JD. 1992. Resurrection and revision
of Hesperevax (Asteraceae: Inuleae). – Syst. Bot.
17: 293-310.
Moreno Cameno MA. 1979. Números de
cromosomas de especies del género Tolpis Adans. en Macaronesia. –
Bot. Macaronésica 5: 61-65.
Moret J, Couderc H, Gorenflot R, Hubac J-M.
1988. La variation morphologique des taxons marocains du genre
Ornithogalum, sous-genre Heliocharmos: une étude
biométrique. – Can. J. Bot. 66: 2178-2186.
Morgan DR. 1993. A molecular systematic study
and taxonomic revision of Psilactis (Asteraceae: Astereae). – Syst. Bot.
18: 290-308.
Morgan DR. 1997. Reticulate evolution in
Machaeranthera (Asteraceae). – Syst Bot. 22:
599-615.
Morgan DR. 2003. nrDNA external transcribed
spacer (ETS) sequence data, reticulate evolution, and the systematics of
Machaeranthera (Asteraceae). – Syst. Bot. 28:
179-190.
Morgan DR, Hartman RL. 2003. A synopsis of
Machaeranthera (Asteraceae: Astereae), with
recognition of segregate genera. – Sida 20: 1387-1416.
Morgan DR, Hollan B. 2012. Systematics of
Symphyotrichinae (Asteraceae:
Astereae): disagreements between two nuclear regions suggest a complex
evolutionary history. – Syst. Bot. 37: 818-832.
Morgan DR, Simpson BB. 1992. A systematic
study of Machaeranthera (Asteraceae) and related groups using
restriction site analysis of chloroplast DNA. – Syst. Bot. 17: 511-531.
Morgan DR, Korn R-L, Mugleston SL. 2009.
Insights into reticulate evolution in Machaerantherinae (Asteraceae: Astereae): 5S ribosomal
RNA spacer variation, estimating support for incongruence, and constructing
reticulate phylogenies. – Amer. J. Bot. 96: 920-932.
Morin N. 1980. Systematics of the annual
California campanulas (Campanulaceae). – Madroño 27:
149-163.
Morin N. 1983. Systematics of
Githopsis (Campanulaceae). – Syst. Bot. 8:
436-468.
Morris KE, Lammers TG. 1997. Circumscription
of Codonopsis and the allied genera Campanumoea and
Leptocodon (Campanulaceae:
Campanuloideae) I. Palynological data. – Bot. Bull Acad. Sin. 38: 277-284.
Morrison DA. 1986. Taxonomic and
nomenclatural notes on Lechenaultia R. Br. (Goodeniaceae). – Brunonia 9:
1-28.
Morrison DA. 1987a. Lechenaultia.
– Aust. Plants 14: 95-114.
Morrison DA. 1987b. The phytogeography,
ecology and conservation status of Lechenaultia R. Br. (Goodeniaceae). – Kingia 1:
85-133.
Morrison DA. 1988. Notes on the fruits of
Lechenaultia (Goodeniaceae), with a new species
from northern Australia. – Telopea 3: 159-166.
Morrison DA. 1989. The genus
Anthotium (Goodeniaceae). – Nuytsia 7:
49-58.
Morrison DA, George A. 2004. The genus
Lechenaultia. – Curtis’s Bot. Mag. 21: 106-131.
Mort ME, Crawford DJ, Santos-Guerra A,
Francisco-Ortega J, Esselman EJ, Wolfe AD. 2003. Relationships among the
Macaronesian members of Tolpis (Asteraceae: Lactuceae) based upon
analyses of inter simple sequence repeat (ISSR) markers. – Taxon 52:
511-518.
Mort ME, Crawford DJ, Fairfield KN. 2004.
Phylogeny and character evolution in California Coreopsis (Asteraceae): insights from morphology
and from sequences of the nuclear and plastid genomes. – Syst. Bot. 29:
781-789.
Mort ME, Randle CP, Kimball RT, Tadesse M,
Crawford DJ. 2008. Phylogeny of Coreopsideae (Asteraceae) inferred from nuclear and
plastid DNA sequences. – Taxon 57: 109-120.
Morton JK. 1977. A cytological study of the
Compositae (excluding Hieracium and Taraxacum) of the British
Isles. – Watsonia 11: 211-223.
Morton JK. 1981. Chromosome numbers in
Compositae from Canada and the U.S.A. – Bot. J. Linn. Soc. 82: 357-368.
Mozaffarian V, Ghahreman A. 2002. Three new
species of Echinops (Compositae, Cynareae) from Iran. – Bot. J.
Linn. Soc. 140: 181-186.
Muchhala N. 2003. Exploring the boundary
between pollination syndromes: bats and hummingbirds as pollinators of
Burmeistera cyclostigmata and B. tenuiflora. – Oecologia
134: 373-380.
Muchhala N. 2006. The pollination biology of
Burmeistera (Campanulaceae): specialization and
syndromes. – Amer. J. Bot. 93: 1081-1089.
Muchhala N, Lammers TG. 2005. A new species
of Burmeistera (Campanulaceae: Lobelioideae) from
Ecuador. – Novon 15: 176-179.
Mueller F von. 1878. Observations on the
genus Phyllachne. – J. Bot. 16: 173-174.
Mueller F von. 1884. Definition of the
characteristics of a new Goodeniaceous plant. – Melbourne Chem. Druggist,
April 1884.
Müller J. 2006. Systematics of
Baccharis (Compositae-Astereae) in Bolivia, including an overview of
the genus. – Syst. Bot. Monogr. 76: 1-341.
Munz PA. 1924. A revision of the genus
Nemacladus (Campanulaceae). – Amer. J. Bot.
11: 233-248.
Murata J. 1992. Systematic implication of
seed coat morphology in Lobelia (Campanulaceae-Lobelioideae). –
J. Fac. Sci. Univ. Tokyo, Sect. III, 15: 155-172.
Murata J. 1995. A revision of infrageneric
classification of Lobelia (Campanulaceae-Lobelioideae) with
special reference to seed coat morphology. – J. Fac. Sci. Univ. Tokyo, Sect.
III, 15: 349-371.
Muschler R. 1909. Systematische und
pflanzengeographische Gliederung der afrikanischen Senecio-Arten. –
Engl. Bot. Jahrb. Syst. 43: 1-74.
Muschler R. 1911. Compositae africanae novae
I. – Engl. Bot. Jahrb. Syst. 46: 51-124.
Nakai T. 1937. Leibnitzia. – J.
Jap. Bot. 13: 852-853.
Napp-Zinn K, Eble M. 1978. Beiträge zur
systematischen Anatomie der Anthemideae: die Spaltöffnungsapparate. – Plant
Syst. Evol. 130: 167-190.
Napp-Zinn K, Eble M. 1980. Beiträge zur
systematischen Anatomie der Asteraceae-Anthemideae: die Trichome.
– Plant Syst. Evol. 136: 169-207.
Negodi G. 1938. Cariologia del genere
Urospermum (Compositae, Liguliflorae-Leontodontinae). – Arch. Bot.
(Forli) 14: 1-19.
Nemirovich-Danchenko EN, Lobova TA. 1998. The
seed coat structure in some representatives of the order Hydrangeales. – Bot.
Žurn. 83: 1-9. [In Russian]
Nesom GL. 1978. Machaeranthera
odyssei (Compositae): a unique new species from Mexico. – Syst. Bot. 3:
218-225.
Nesom GL. 1980. A revision of the epappose
species of Erigeron (Compositae-Astereae). – Ph.D. diss., University
of North Carolina, Chapel Hill, North Carolina.
Nesom GL. 1982. Systematics of the
Erigeron rusbyi group (Asteraceae) and delimitation of sect.
Peregrinus. – Syst. Bot. 7: 457-470.
Nesom GL. 1983. Biology and taxonomy of
American Leibnitzia (Asteraceae: Mutisieae). – Brittonia
35: 126-139.
Nesom GL. 1988a. Synopsis of
Chaetopappa (Compositae-Astereae) with a new species and the inclusion
of Leucelene. – Phytologia 64: 448-456.
Nesom GL. 1988b. Baccharis sect.
Baccharidastrum (Compositae-Astereae), including two monoecious and
one dioecious species. – Phytologia 65: 169-173.
Nesom GL. 1989a. Taxonomy of
Erigeron sect. Polyactis (Compositae: Astereae). –
Phytologia 66: 415-455.
Nesom GL. 1989b. The separation of
Trimorpha (Compositae-Astereae) from Erigeron. – Phytologia
67: 61-66.
Nesom GL. 1989c. Infrageneric taxonomy of New
World Erigeron (Compositae: Astereae). – Phytologia 67: 67-93.
Nesom GL. 1989d. A new combination in
Stenotus (Compositae: Astereae). – Phytologia 67: 113-114.
Nesom GL. 1990a. An additional species of
Gnaphaliothamnus (Asteraceae: Inuleae) and further
evidence for the integrity of the genus. – Phytologia 69: 1-3.
Nesom GL. 1990b. Taxonomic summary of
Ericameria (Asteraceae:
Astereae), with the inclusion of Haplopappus sects. Macronema
and Asiris. – Phytologia 68: 144-155.
Nesom GL. 1990c. Taxonomic status of
Gamochaeta (Asteraceae:
Inuleae) and the species of the United States. – Phytologia 68: 186-198.
Nesom GL. 1990d. Taxonomy of the genus
Laennecia (Asteraceae:
Astereae). – Phytologia 68: 205-228.
Nesom GL. 1990e. Further definition of
Conyza (Asteraceae:
Astereae). – Phytologia 68: 229-233.
Nesom GL. 1990f. Taxonomic summary of
Omalotheca (Asteraceae:
Inuleae). – Phytologia 68: 241-246.
Nesom GL. 1990g. Taxonomy of
Gnaphaliothamnus (Asteraceae: Inuleae). – Phytologia
68: 366-381.
Nesom GL. 1990h. Taxonomy of the Erigeron
pringlei group (Asteraceae:
Astereae). – Phytologia 69: 227-235.
Nesom GL. 1990i. Taxonomy of
Heterotheca sect. Heterotheca (Asteraceae-Astereae) in Mexico, with
comments on the taxa of the United States. – Phytologia 69: 282-294.
Nesom GL. 1991a. Taxonomy of Isocoma
(Compositae: Astereae). – Phytologia 70: 69-114.
Nesom GL. 1991b. Union of Bradburia with
Chrysopsis (Asteraceae:
Astereae), with a phylogenetic hypothesis for Chrysopsis. –
Phytologia 71: 109-121.
Nesom GL. 1991c. Transfer of Aster
kingii to Tonestus (Asteraceae: Astereae). – Phytologia
71: 122-127.
Nesom GL. 1991d. Tomentaurum (Asteraceae: Astereae), a new genus of
goldenaster from Chihuahua, Mexico. – Phytologia 71: 128-131.
Nesom GL. 1991e. Transfer of Heterotheca
bartletii to Osbertia (Asteraceae: Astereae). – Phytologia
71: 132-135.
Nesom GL. 1991f. A phylogenetic hypothesis
for the goldenasters (Asteraceae:
Astereae). – Phytologia 71: 136-151.
Nesom GL. 1991g. Two new species of
Archibaccharis (Asteraceae: Astereae) from Mexico
with a reevaluation of sectional groupings in the genus. – Phytologia 71:
152-159.
Nesom GL. 1991h. Redefinition of
Hesperodoria (Asteraceae:
Astereae) and the segregation of Columbiadoria, a new monotypic genus
from the western United States. – Phytologia 71: 244-251.
Nesom GL. 1991i. Morphological definition of
the Gutierrezia group (Asteraceae: Astereae). – Phytologia
71: 252-262.
Nesom GL. 1993. Taxonomic infrastructure of
Solidago and Oligoneuron (Asteraceae: Astereae) and
observations on their phylogenetic position. – Phytologia 75: 1-44.
Nesom GL. 1994a. Erigeron
pattersonii (Asteraceae:
Astereae), a new species from Nuevo Leon, Mexico. – Phytologia 76: 96-100.
Nesom GL. 1994b. Taxonomic dispersal of
Australian Erigeron (Asteraceae: Astereae). – Phytologia
76: 143-159.
Nesom GL. 1994c. Subtribal classification of
the Astereae (Asteraceae). –
Phytologia 76: 193-274.
Nesom GL. 1994d. Review of the taxonomy of
Aster sensu lato (Asteraceae: Astereae), emphasizing
New World species. – Phytologia 77: 141-297.
Nesom GL. 1998 [2000]. Full constitution of
the Australian genus Pappochroma (Asteraceae: Astereae). – Phytologia
85: 276-279.
Nesom GL. 2000a. New subtribes for North
American Astereae (Asteraceae).
– Sida 19: 263-268.
Nesom GL. 2000b. Generic conspectus of the
tribe Astereae in North America, Central America, the Antilles, and Hawaii. –
Sida Bot. Misc. 20: i-viii, 1-100.
Nesom GL. 2001. New combinations in
Chionolaena (Asteraceae:
Gnaphalieae). – Sida 19: 849-852.
Nesom GL. 2005. Infrageneric classification
of Liatris (Asteraceae:
Eupatorieae). – Sida 21: 1305-1321.
Nesom GL, Morgan DR. 1990. Reinstatement of
Tonestus (Asteraceae:
Astereae). – Phytologia 68: 174-180.
Nesom GL, Noyes RD. 1999. Notes on sectional
delimitations in Erigeron (Asteraceae: Astereae). – Sida 18:
1161-1165.
Nesom GL, Noyes RD. 2000.
Batopilasia (Asteraceae:
Astereae), a new genus from Chihuahua, Mexico. – Sida 19: 79-84.
Nesom GL, Suh Y, Morgan DR, Simpson BB. 1990.
Xylothamia (Asteraceae-Astereae), a new genus
related to Euthamia. – Sida 14: 101-116.
Nesom GL, Robinson H, Granda Paucar A. 2001.
A new species of Chiliotrichopsis (Asteraceae: Astereae) from Peru. –
Brittonia 53: 430-434.
Newcomb W. 1973. The development of the
embryo sac of sunflower Helianthus annuus before fertilization. –
Can. J. Bot. 51: 863-878.
Nguyen Thi Nghia, Sedmera P, Klásek A, Boeva
A, Drjanovska L, Dolejš L, Šantavý F. 1976. Bulgarsenine and retroisosenine,
alkaloids from Senecio nemorensis L. var. bulgaricus (Vel.)
Stoj. et Stef. – Coll. Czech. Chem. Commun. 41: 2952-2963.
Nguyen Van Thuan 1969. Campanulaceae. – In: Aubréville
A (ed), Flore du Cambodge, du Laos et du Vietnam 9, Muséum National
d’Histoire Naturelle, Paris.
Nic Lughadha EM, Parnell JAN. 1989.
Heterostyly and gene-flow in Menyanthes trifoliata L. (Menyanthaceae). – Bot. J. Linn.
Soc. 100: 337-354.
Nicola MV, Johnson LA, Pozner R. 2014.
Geographic variation among closely related, highly variable species with a wide
distribution range: the South Andean-Patagonian Nassauvia subgenus
Strongyloma (Asteraceae,
Nassauvieae). – Syst. Bot. 39: 331-348.
Nicolson DH. 1980. Summary of cytological
information on Emilia and the taxonomy of four Pacific taxa of
Emilia (Asteraceae:
Senecioneae). – Syst. Bot. 5: 391-407.
Nicolson DH. 1996. Proposal to conserve the
name Lagenophora (Compositae) with a conserved spelling. – Taxon 45:
341-342.
Nicolson DH. 1997. Proposal to conserve the
name Petrobium (Compositae: Heliantheae). – Taxon 46: 807-808.
Nijs HCM den, Menken SBJ. 1996. Relations
between breeding system, ploidy level and taxonomy in some advanced sections of
Taraxacum. – In: Hind DJN, Beentje HJ (eds), Compositae:
systematics. Proceedings of the International Compositae Conference, Kew, 1994,
vol. 1, Royal Botanic Gardens, Kew pp. 665-677.
Nikolakaki A, Christodoulakis NS. 2004. Leaf
structure and cytochemical investigation of secretory tissues in Inula
viscosa. – Bot. J. Linn. Soc. 144: 437-448.
Nilsson S, Ornduff R. 1973. Menyanthaceae Dum. – World
Pollen and Spore Flora 2, Almqvist & Wiksell, Stockholm, pp. 1-20.
Nishino E. 1983. Corolla tube formation in
the Tubiflorae and Gentianales. – Bot. Mag. (Tokyo) 96: 223-243.
Nordenstam B. 1961. Two new Compsitae from
Southern Africa. – Bot. Not. 119: 365-370.
Nordenstam B. 1966a. Two new Compositae from
Southern Africa. – Bot. Not. 119: 365-370.
Nordenstam B. 1966b. Euryops in
South West Africa. – Bot. Not. 119: 475-485.
Nordenstam B. 1967a. New species of
Felicia and Pentzia (Compositae) from the Brandberg, South
West Africa. – Bot. Not. 120: 196-201.
Nordenstam B. 1967b. Chromosome numbers in
South African Compositae. – Aquilo, Ser. Bot. 6: 219-226.
Nordenstam B. 1967c. Chromosome numbers in
Othonna (Compositae). – Bot. Not. 120: 297-304.
Nordenstam B. 1968a. The genus
Euryops I. Taxonomy. – Opera Bot. 20: 1-409.
Nordenstam B. 1968b. The genus
Euryops II. Aspects of morphology and cytology. – Bot. Not. 121:
209-232.
Nordenstam B. 1969a. Chromosome studies on
South African vascular plants. – Bot. Not. 122: 398-408.
Nordenstam B. 1969b. Phytogeography of the
genus Euryops (Compositae). A contribution to the phytogeography of
Southern Africa. – Opera Bot. 23: 1-77.
Nordenstam B. 1971a. New South African
Compositae. – Bot. Not. 124: 9-15.
Nordenstam B. 1971b. Cytogeography of the
genus Hedypnois. – Bot. Not. 124: 483-489.
Nordenstam B. 1972. Chromosome numbers in
some Compositae from Egypt. – Bot. Not. 125: 393-396.
Nordenstam B. 1975 [1976].
Lamprocephalus B. Nord., a new senecionoid genus from South Africa.
– Bot. Not. 128: 323-326.
Nordenstam B. 1976. Re-classification of
Chrysanthemum L. in South Africa. – Bot. Not. 129: 137-165.
Nordenstam B. 1977a. Senecioneae and Liabeae
– systematic review. – In: Heywood VH, Harborne JB, Turner BL (eds), The
biology and chemistry of the Compositae 2, Academic Press, London, pp.
799-830.
Nordenstam B. 1977b. Scyphopappus B.
Nord. is Argyranthemum Webb ex Sch. Bip. (Compositae-Anthemideae). –
Bot. Not. 129: 427-428.
Nordenstam B. 1977c. A review of the Andean
genus Cacosmia H.B.K. (Compositae-Liabeae). – Bot. Not. 130:
279-286.
Nordenstam B. 1978. Taxonomic studies in the
tribe Senecioneae (Compositae). – Opera Bot. 44: 1-83.
Nordenstam B. 1979. Adenanthellum
nom. nov. (Compositae-Anthemideae). – Bot. Not. 132: 160.
Nordenstam B. 1987. Notes on S African
Anthemideae (Compositae). – Opera Bot. 92: 147-151.
Nordenstam B. 1994. New combinations in the
Calenduleae. – Compositae Newslett. 25: 46-49.
Nordenstam B. 1996. Recent revision of
Senecioneae and Calenduleae systematics. – In: Hind DJN, Beentje HJ (eds),
Compositae: systematics. Proceedings of the International Compositae
Conference, Kew, 1994, vol. 1, Royal Botanic Gardens, Kew, pp. 591-596.
Nordenstam B. 1997. Nomenclatural notes on
Ecuadorian Senecioneae. – Compositae Newslett. 30: 46-49.
Nordenstam B. 2002. Capelio B.
Nord., a new name for a South African genus of the Senecioneae, and the
description of a new species. – Compositae Newslett. 38: 71-78.
Nordenstam B. 2003a. Alciope versus
Capelio – a nomenclatural ordeal. – Compositae Newslett. 39:
48-51.
Nordenstam B. 2003b. Recent progress in
Senecioneae taxonomy. – Compositae Newslett. 40: 26.
Nordenstam B. 2004. Two new species of
Osteospermum (Compositae-Calenduleae) from Southwestern Cape Province,
South Africa. – Edinburgh J. Bot. 60: 259-265.
Nordenstam B. 2006a. Additions to the genus
Jacobaea Mill. (Compositae-Senecioneae). – Compositae Newslett. 44:
12-13.
Nordenstam B. 2006b. New combinations in
Nordenstamia (Compositae-Senecioneae) from Argentina, Bolivia, Peru
and Ecuador. – Compositae Newslett. 44: 19-23.
Nordenstam B. 2006c. Canariothamnus
B. Nord., a new genus of the Compositae-Senecioneae, endemic to the Canary
Islands. – Compositae Newslett. 44: 24-31.
Nordenstam B. 2006d. Generic revisions in the
tribe Calenduleae (Compositae). – Compositae Newslett. 44: 38-49.
Nordenstam B. 2006e. New genera and
combinations in the Senecioneae of the Greater Antilles. – Compositae
Newslett. 44: 50-73.
Nordenstam B. 2006f. New genera and
combinations in the Senecioneae of the Greater Antilles. – Compositae
Newslett. 44: 74-93.
Nordenstam B. 2006g. Ignurbia, a new
genus of the Asteraceae-Senecioneae from
Hispaniola. –Willdenowia 36 (Spec. issue): 463-468.
Nordenstam B, Cron GV. 2006.
Oresbia, a new South African genus of the Asteraceae, Senecioneae. – Novon
16: 216-223.
Nordenstam B, El-Ghazaly G. 1977. Floral
micromorphology and pollen ultrastructure in some Centaureinae (Compositae)
mainly from Egypt. – Publ. Cairo Univ. Herb. 7-8: 143-155.
Nordenstam B, Funk VA. 2009. Corymbieae. –
In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and
biogeography of Compositae, International Association for Plant Taxonomists,
Wien, pp. 487-491.
Nordenstam B, Greuter W. 2006. Jacobaea Mill.
– In: Greuter W, Raab-Straube E von (eds), Euro+Med Notulae 2, Willdenowia
36: 707-717, p. 711-713.
Nordenstam B, Källersjö M. 2009.
Calenduleae. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 527-538.
Nordenstam B, Pelser PB. 2005.
Dauresia and Mesogramma: one new and one resurrected genus of
the Asteraceae-Senecioneae from
Southern Africa. – Compositae Newslett. 42: 74-88.
Nordenstam B, Pruski JF. 1995. Additions to
Dorobaea and Talamancalia (Compositae-Senecioneae). – Comp.
Newsl. 27: 31-42.
Nordenstam B, Källersjö M, Eldenäs P.
2006. Nephrotheca, a new monotypic genus of the Compositae-Calenduleae
from the Southwestern Cape Province. – Compositae Newslett. 44: 32-37.
Nordenstam B, Clark VR, Devos N, Barker NP.
2009. Two new species of Euryops (Asteraceae: Senecioneae) from the
Sneeuberg, Eastern Cape Province, South Africa. – South Afr. J. Bot. 75:
145-152.
Nordenstam B, Pelser PB, Watson LE. 2009.
Lomanthus, a new genus of the Compositae-Senecioneae from Ecuador,
Peru, Bolivia, and Argentina. – Comp. Newsl. 47: 33-41.
Nordenstam B, Pelser PB, Kadereit JW, Watson
LE. 2009. Senecioneae. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 503-525.
Norlindh T. 1943. Studies in the Calenduleae
I. Monograph of the genera Dimorphotheca, Castalis,
Osteospermum, Gibbaria and Chrysanthemoides. –
Gleerup, Lund, Sweden.
Norlindh T. 1946. Studies in the Calenduleae
II. Phytogeography and interrelation. – Bot. Not. 81: 471-509.
Norlindh T. 1960. Additions to the monograph
on Osteospermum. – Bot. Not. 113: 385-399.
Norlindh T. 1962. Studies in Calendula
maderensis DC. with a discussion on the delimitation of Calendula
L. from Gibbaria Cass. and Osteospermum L. – Bot. Not. 115:
437-445.
Norlindh T. 1963. Chromosome numbers in the
Calenduleae I. – Bot. Not. 116: 193-209.
Norlindh T. 1977a. Arctot[id]eae –
systematic review. – In: Heywood VH, Harborne JB, Turner BL (eds), The
biology and chemistry of the Compositae, Academic Press, London, pp.
943-959.
Norlindh T. 1977b. Calenduleae – systematic
review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae, Academic Press, London, pp. 961-987.
Norlindh T. 1977c. Garuleum
subgen.Rutidocarpaea, a monotypic subgenus showing achene dimorphism.
– Bot. Not. 130: 377-380.
Noshiro S, Baas P. 1998. Systematic wood
anatomy in Cornaceae and
allies. – IAWA J. 19: 43-97.
Nowicke JW, Shetler SG, Morin N. 1992. Exine
structure of pantoporate Campanula (Campanulaceae) species. – Ann.
Missouri Bot. Gard. 79: 65-80.
Noyes RD. 2000. Biogeographical and
evolutionary insights on Erigeron and allies (Asteraceae) from ITS sequence data.
– Plant Syst. Evol. 220: 93-114.
Noyes RD, Rieseberg LH. 1999. ITS sequence
data support a single origin for North American Astereae (Asteraceae) and reflect deep
geographic divisions within Aster s.l. – Amer. J. Bot. 86:
398-412.
Nylinder S, Anderberg AA. 2015. Phylogeny of
the Inuleae (Asteraceae) with
special emphasis on the Inuleae-Plucheinae. – Taxon 64: 110-130.
Nylinder S, Cronholm B, Lange PJ de, Walsh N,
Anderberg AA. 2013. Species tree phylogeny and character evolution in the genus
Centipeda (Asteraceae):
evidence from DNA sequences from coding and non-coding loci from the plastid
and nuclear genomes. – Mol. Phylogen. Evol. 68: 239-250.
Nylinder S, Lemey P, De Bruyn M, Suchard MA,
Pfeil BE, Walsh N, Anderberg AA. 2014. On the biogeography of
Centipeda: a species-tree diffusion approach. – Syst. Biol. 63:
178-191.
Oberhänsli T, Huber W. 1993. Zur
Chemotaxonomie von Erigeron-Arten (Compositae) der Alpen. – Ber.
Geobot. Inst. ETH, Stiftung Rübel, Zürich 59: 124-136.
Oberprieler C. 1994. Anthemis
gharbensis (Compositae, Anthemideae), a new species from NW Morocco. –
Willdenowia 24: 83-89.
Oberprieler C. 1998. The systematics of
Anthemis L. (Compositae, Anthemideae) in W and C North Africa. –
Bocconea 9: 5-328.
Oberprieler C. 2001. Phylogenetic
relationships in Anthemis (Compositae, Anthemideae) based on nrDNA ITS
sequence variation. – Taxon 50: 745-762.
Oberprieler C. 2002. A phylogenetic analysis
of Chamaemelum Miller (Compositae, Anthemideae) and related genera
based upon nrDNA ITS and cpDNA trnL/trnF IGS sequence
variation. – Bot. J. Linn. Soc. 138: 255-273.
Oberprieler C. 2004a. On the taxonomic status
and the phylogenetic relationships of some unispecific Mediterranean genera of
Compositae-Anthemideae I. Brocchia, Endopappus, and
Heliocauta. – Willdenowia 34: 39-57.
Oberprieler C. 2004b. On the taxonomic status
and the phylogenetic relationships of some unispecific Mediterranean genera of
Compositae-Anthemideae II. Daveaua, Leucocyclus, and
Nananthea. – Willdenowia 34: 341-350.
Oberprieler C. 2005. Temporal and spatial
diversification of Circum-Mediterranean Compositae-Anthemideae. – Taxon 54:
951-966.
Oberprieler C, Vogt R. 1999 [2000]. Notes on
some species of Anthemis (Compositae, Anthemideae) in Cyprus. –
Bocconea 11: 89-104.
Oberprieler C, Vogt R. 2000. The position of
Castrilanthemum Vogt & Oberprieler and the phylogeny of
Mediterranean Anthemideae (Compositae) as inferred from nrDNA ITS and cpDNA
trnL/trnF IGS sequence variation. – Plant Syst. Evol. 225:
145-170.
Oberprieler C, Himmelreich S, Vogt R. 2007. A
new subtribal classification of the tribe Anthemideae (Compositae). –
Willdenowia 37: 89-114.
Oberprieler C, Himmelreich S, Källersjö M,
Vallès J, Watson LE, Vogt R. 2009. Anthemideae. – In: Funk VA, Susanna A,
Stuessy TF, Bayer RJ (eds), Systematics, evolution, and biogeography of
Compositae, International Association for Plant Taxonomists, Wien, pp.
631-666.
Ochsmann J. 2000. Morphologische und
molekularsystematische Untersuchungen an der Centaurea stoebe
L.-Gruppe (Asteraceae-Cardueae) in
Europa. – Diss. Bot. 324.
Okada M, Lowrey TK, Whitkus R. 2000.
Quantitative morphological variation in Tetramolopium (Asteraceae) in Hawaii and the Cook
Islands. – Plant Syst. Evol. 22: 1-13.
Oliva Brañas M, Vallès Xirau J. 1991.
Contribution to the cytotaxonomical knowledge of the genus Artemisia
L.: Giemsa C-banded karyotypes of some taxa. – Bot. Chron. 10: 737-740.
Oliva Brañas M, Vallès Xirau J. 1994.
Karyological studies in some taxa of the genus Artemisia (Asteraceae). – Can. J. Bot. 72:
1126-1135.
Øllgaard H. 1983. Hamata, a new
section of Taraxacum (Asteraceae). – Plant Syst. Evol.
141: 199-217.
Ono M. 1967a. Chromosome number of
Scalesia (Compositae), an endemic genus of the Galápagos Islands. –
J. Jap. Bot. 42: 353-360.
Ono M. 1967b. The systematic position of
Scalesia from the viewpoint of chromosome number. – Not. Galápagos
9-10: 16-17.
Ono M. 1971. Chromosome number of
Scalesia (Compositae), an endemic genus of the Galápagos Islands 2.
– J. Jap. Bot. 46: 327-334.
Orchard AE. 1981. The generic limits of
Ixodia R. Br. ex Ait. (Compositae-Inuleae). – Brunonia 4:
185-197.
Orchard AE. 1992. Ammobium and
Nablonium (Asteraceae-Gnaphalieae) – an
alternative view. – Telopea 5: 1-12.
Orchard AE. 2004a. A reassessment of the
genus Haeckeria (Asteraceae: Gnaphalieae), with
definition of new species in Cassinia. – Aust. Syst. Bot. 17:
447-467.
Orchard AE. 2004b. A revision of
Cassinia (Asteraceae:
Gnaphalieae) in Australia 1. Introduction and generic and infrageneric
considerations. – Aust. Syst. Bot. 17: 469-481.
Orchard AE. 2004c. A revision of
Cassinia (Asteraceae:
Gnaphalieae) in Australia 2. Sections Complanatae and
Venustae. – Aust. Syst. Bot. 17: 505-533.
Orchard AE. 2004d. A revision of
Cassinia (Asteraceae:
Gnaphalieae) in Australia 3. Section Leptocephalae. – Aust. Syst.
Bot. 17: 535-565.
Orchard AE. 2005a. Proposal to conserve
Cassinia R. Br. (1817) nom. cons. (Asteraceae) against an additional
neme, Ozothamnus, or to change its date of publication to
Cassinia R. Br. (1813). – Taxon 54: 199-201.
Orchard AE. 2005b. A revision of
Cassinia (Asteraceae:
Gnaphalieae) in Australia 4. Section Costatae. – Aust. Syst. Bot.
18: 455-471.
Orchard AE. 2005c. Paenula storyi, a
new genus and species related to Ixodia and Haeckeria (Asteraceae: Gnaphalieae). – Telopea
11: 1-9.
Orchard AE. 2006. A revision of
Cassinia (Asteraceae:
Gnaphalieae) in Australia 5. Additional taxa in Section Leptocephalae.
– Aust. Syst. Bot. 19: 183-191.
Orchard AE. 2009. A revision of
Cassinia (Asteraceae:
gnaphalieae) in Australia 6. Section Cassinia. – Aust. Syst. Bot.
22: 344-376.
Orchard AE. 2011. A review of Australian
Adenostemma J. R. Forst. & G. Forst. (Asteraceae: Eupatorieae). – Telopea
13: 341-348.
Orchard AE. 2013. The
Wollastonia/Melanthera/Wedelia generic complex (Asteraceae: Ecliptinae), with
particular reference to Australia and Malesia. – Nuytsia 23: 337-466.
Orchard AE. 2017. A revision of
Cassinia (Asteraceae:
Gnaphalieae) in Australia. 7. Cassinia subgenus Achromolaena.
– Aust. Syst. Bot. 30: 337-370.
Orchard AE, Cross EW. 2012. A revision of the
Australian endemic genus Pentalepis (Asteraceae: Ecliptinae). – Nuytsia
22: 371-392.
Orchard AE, Cross EW. 2013. A revision of the
Australian species of Eclipta (Asteraceae: Ecliptinae), with
discussion of extra-Australian taxa. – Nuytsia 23: 43-62.
Orchard AE, Wilson AJG. 2004. Cassinia
glossophylla Cas. is Ozothamnus leptophyllus (G. Forst.) Breitw.
& J. M. Ward (Asteraceae). –
Aust. Syst. Bot. 17: 567-570.
Ornduff R. 1963. Experimental studies in two
genera of Helenieae (Compositae): Blennosperma and Lasthenia.
– Quart. Rev. Biol. 38: 141-150.
Ornduff R. 1966a. A biosystematic study of
the goldfield genus Lasthenia (Compositae: Helenieae). – Univ.
Calif. Publ. Bot. 40: 1-92.
Ornduff R. 1966b. The origin of dioecism from
heterostyly in Nymphoides (Menyanthaceae). – Evolution 20:
309-314.
Ornduff R. 1969. Neotropical
Nymphoides (Menyanthaceae): Meso-American and
West Indian species. – Brittonia 21: 346-352.
Ornduff R. 1970. Cytogeography of
Nymphoides (Menyanthaceae). – Taxon 19:
715-719.
Ornduff R. 1974. Cytotaxonomic observations
on Villarsia (Menyanthaceae). – Aust. J. Bot.
22: 513-516.
Ornduff R. 1982. Heterostyly and
incompatibility in Villarsia capitata (Menyanthaceae). – Taxon 31:
495-497.
Ornduff R. 1986. Comparative fecundity and
population composition of heterostylous and non-heterostylous species of
Villarsia (Menyanthaceae) in Western
Australia. – Amer. J. Bot. 73: 282-286.
Ornduff R. 1988. Distyly and monomorphism in
Villarsia (Menyanthaceae): some evolutionary
considerations. – Ann. Missouri Bot. Gard. 75: 761-767.
Ornduff R. 1990. A new species of
Villarsia (Menyanthaceae) from the Porongorup
Range, Western Australia. – Syst. Bot. 15: 216-220.
Ornduff R. 1994. Villarsia
cambodiana (Menyanthaceae)
in southeastern Asia. – Nord. J. Bot. 14: 674-648.
Ornduff R. 1996. The breeding system of
Villarsia exaltata (Menyanthaceae), a distylous
species. – Telopea 6: 805-811.
Ornduff R. 1999. A new species of
Villarsia (Menyanthaceae) from South Africa.
– Novon 9: 407-409.
Ornduff R. 2001. A(nother) new species of
Villarsia (Menyanthaceae) from South Africa.
– Novon 11: 437-439.
Ornduff R, Chuang TI. 1988. Chromosome
numbers of Western Australian species of Villarsia (Menyanthaceae). – Plant Syst.
Evol. 161: 49-52.
Ornduff R, Raven PH, Kyhos DW, Kruckeberg A.
1963. Chromosome numbers in Compositae III. Senecioneae. – Amer. J. Bot. 50:
131-139.
Ornduff R, Mosquin T, Kyhos DW, Raven PH.
1967. Chromosome numbers in Compositae VI. Senecioneae II. – Amer. J. Bot.
54: 205-213.
Ornduff R, Saleh NAM, Bohm BA. 1973. The
flavonoids and affinities of Blennosperma and Crocidium
(Compositae). – Taxon 22: 407-412.
Ortega A, Martínez R, Garcia CL. 1980. Los
diterpenes de Stevia salicifolia – estructura del stevinsol and
salicifoliol. – Rev. Latinoamer. Quim. 11: 45-48.
Ortiz S. 1999. Three new scapose species of
Vernonia Schreb. (Asteraceae, Vernonieae) from Angola.
– Can. J. Bot. 77: 877-883.
Ortíz S. 2000. A phylogenetic analysis of
Dicoma Cass. and related genera (Asteraceae: Cichorioideae: Mutisieae)
based on morphological and anatomic characters. – Ann. Missouri Bot. Gard.
87: 459-481.
Ortíz S. 2001. The reinstatement of the
genus Macledium Cass. (Asteraceae, Mutisieae): morphological
and phylogenetic arguments. – Taxon 50: 733-744.
Ortíz S. 2005. Nomenclatural notes on the
genus Anisopappus Hook. & Arn. (Asteraceae, Inuleae). – Nova Acta
Ci. Compostelana, Biol. 14: 89-92.
Ortíz S. 2006. Systematics of
Cloiselia (Asteraceae,
Mutisieae s.l.), a reinstated Madagascan genus. – Syst. Bot. 31: 421-431.
Ortíz S. 2007. A new species of
Felicia L. (Asteraceae,
Astereae) from South Africa. – Bot. J. Linn. Soc. 154: 545-548.
Ortíz S. 2009a. Eight new species of
Marasmodes (Asteraceae,
Anthemideae) from South Africa. – Bot. J. Linn. Soc. 159: 330-342.
Ortíz S. 2009b. Tarchonantheae
(Carduoideae). – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 279-285.
Ortíz S. 2009c. Oldenburgieae (Carduoideae).
– In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution,
and biogeography of Compositae, International Association for Plant
Taxonomists, Wien, pp. 287-291.
Ortíz S. 2010. Cardosoa, a new
genus of the subtribe Anisopappinae (Athroismeae, Asteraceae). – An. Jard. Bot.
Madrid 67: 7-11.
Ortíz S, Coutinho AP. 2001.
Achyrothalamus reduced to Erythrocephalum (Asteraceae, Mutisieae). – Taxon 50:
389-403.
Ortíz S, Netnou NC. 2005. A new species of
Dicoma (Asteraceae,
Mutisieae) from South Africa. – Bot. J. Linn. Soc. 147: 509-513.
Ortíz S, Oubiña JR. 1996. Two new species
of Dicoma (Asteraceae)
from Somalia and Ethiopia. – Nord. J. Bot. 16: 277-281.
Ortíz S, Paiva JAR. 1995. Notes on African
species of the genus Anisopappus Hook. & Arn. (Asteraceae). – Bot. J. Linn. Soc.
117: 39-46.
Ortíz S, Pulgar I. 2002. A new species of
Dicoma Cass. (Asteraceae:
Mutisieae) from Namibia. – Bot. J. Linn. Soc. 139: 317-322.
Ortíz S, Rodríguez-Oubiña J. 1995.
Dicoma hindana (Asteraceae), a new species from
Somalia. – Nord. J. Bot. 15: 187-189.
Ortíz S, Rodríguez-Oubiña J. 1996. A new
taxonomic interpretation of the Dicoma anomala complex (Mutisieae, Asteraceae). – Nord. J. Bot. 16:
583-584.
Ortíz S, Paiva JAR, Rodríguez-Oubiña J.
1996. An outline of the genus Anisopappus Hook. & Arn.
(Compositae). – An. Jard. Bot. Madrid 54: 378-391.
Ortíz S, Rodríguez-Oubiña J, Tadesse M.
1998. A taxonomic revision of Dicoma (Asteraceae, Cichorioideae, Mutisieae)
for the Horn of Africa. – Ann. Missouri Bot. Gard. 85: 440-459.
Ortíz S, Paiva JAR, Pulgar I. 2001. A new
species of Anisopappus (Asteraceae, Inuleae) and its
phylogenetic relationships within the genus. – Nord. J. Bot. 21: 55-62.
Ortíz S, Bonifacino JM, Crisci JV, Funk VA,
Hansen HV, H DJN, Katinas L, Roque N, Sancho G, Susanna A, Tellería MC. 2009.
The basal grade of Compositae: Mutisieae (sensu Cabrera) and Carduoideae. –
In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and
biogeography of Compositae, International Association for Plant Taxonomists,
Wien, pp. 193-213.
Ortíz S, Carbajal R, Serrano M, Coutinho
AXP. 2009. Dicomeae (Carduoideae). – In: Funk VA, Susanna A, Stuessy TF,
Bayer RJ (eds), Systematics, evolution, and biogeography of Compositae,
International Association for Plant Taxonomists, Wien, pp. 267-278.
Ortíz S, Carbajal R, Serrano M,
Rodríguez-Oubiña J, Iglesias I. 2013. Phylogeny of the African Mutisieae s.l.
(Asteraceae) based on
ndhF and trnL-F sequences (cpDNA). – Taxon 62: 525-536.
Osman AK. 2006. Contributions to the pollen
morphology of the tribe Inuleae (subfamily Asteroideae-Compositae) in the flora
of Egypt. – Feddes Repert. 117: 193-206.
Osman AK. 2009. Contributions to the pollen
morphology of tribe Cardueae (Cichorioideae-Compositae). – Feddes Repert.
120: 145-157.
Otocka B, Geszprych A. 2004. Anatomy of the
vegetative organs and secretory structures of Rhaponticum carthamoides
(Asteraceae). – Bot. J. Linn.
Soc. 144: 207-233.
Ownbey GB, Olson WA. 1969. Cytotaxonomic
notes on the species of Cirsium native to the Southeastern United
States. – Rhodora 71: 285-296.
Ownbey GB, Pinkava DJ. 1980. Cirsium
coahuilense (Asteraceae), a
new species from Northern Mexico. – Syst. Bot. 5: 326-328.
Pacheco P, Crawford DJ, Stuessy TF, Silva OM.
1991. Flavonoid evolution in Dendroseris (Compositae, Lactuceae) from
the Juan Fernandez Islands, Chile. – Amer. J. Bot. 78: 534-543.
Pacini E. 1996. Tapetum types in the
Compositae: forms and function. – In: Hind DJN, Beentje HJ (eds), Compositae:
systematics. Proceedings of the International Compositae Conference, Kew, 1994,
Royal Botanic Gardens, Kew, pp. 21-28.
Packer JG. 1972. A taxonomic and
phytogeographical review of some arctic and alpine Senecio species.
– Can. J. Bot. 50: 507-518.
Padilla-González GF, Diazgranados M,
Oliveira TB, Chagas-Paula DA, Da Costa FB. 2018. Chemistry of the subtribe
Espeletiinae (Asteraceae) and its
correlation with phylogenetic data: an in silico chemosystematic approach. –
Bot. J. Linn. Soc. 186: 18-46.
Padin AL, Calviño CI, Ezcurra C. 2015.
Molecular phylogeny of Chuquiraga (Asteraceae-Barnadesioideae):
infrageneric classification and generic affinities. – Syst. Bot. 40:
316-326.
Paiva JAR. 1972. New and little known species
from the Flora Zambesiaca area XXII. Notes on the Inuleae. – Bol. Soc. Brot.
46: 355-385.
Pak J-H. 1991. A taxonomical review of
Ixeris s.l. (Compositae-Lactuceae) – karyological and fruit wall
characteristics. – Korean J. Plant Taxon. 21: 71-82.
Pak J-H. 1993. Taxonomic implications of
fruit wall anatomy and karyology of Crepis sect. Ixeridopsis
(Compositae-Lactuceae). – Korean J. Plant Taxon. 23: 11-20.
Pak J-H, Bremer K. 1945. Phylogeny and
reclassification of the genus Lapsana (Asteraceae: Lactuceae). – Taxon 44:
13-21.
Pak J-H, Kawano S. 1990a. Biosystematic
studies on the genus Ixeris (Compositae-Lactuceae) I. Fruit wall
anatomy and its taxonomic implications. – Acta Phytotaxon. Geobot. 41:
43-60.
Pak J-H, Kawano S. 1990b. Biosystematic
studies on the genus Ixeris (Compositae-Lactuceae) II. Cytologia 55:
553-570.
Pak J-H, Kawano S. 1990c. Biosystematic
studies on the genus Ixeris (Compositae-Lactuceae) III. Fruit wall
anatomy and karyology of Crepidiastrum and Paraixeris, and
their taxonomic implications. – Acta Phytotaxon. Geobot. 41: 109-128.
Pak J-H, Kawano S. 1990. Biosystematic
studies on the genus Ixeris (Compositae-Lactuceae) IV. Taxonomic
treatments and nomenclature. – Mem. Fac. Sci. Kyoto Univ., Ser. B, Biol. 15:
29-60.
Palazzesi L, Barreda V, Tellería MC. 2009.
Fossil pollen grains of Asteraceae
from the Miocene of Patagonia: Barnadesioideae affinity. – Rev. Palaeobot.
Palynol. 155: 83-88.
Palazzesi L, Barreda V, Telleria MC. 2010.
First fossil record of Calyceraceae (Asterales): pollen
evidence from southern South America. – Rev. Palaeobot. Palynol. 158:
236-239.
Paliwal GS, Srivastava LM. 1969 [1970]. The
cambium of Alseuosmia. – Phytomorphology 19: 5-8.
Palm B. 1925. Embryological notes on tropical
Compositae 1. Vernonia chinensis and V. cineraria. – Ann.
Jard. Bot. Buitenzorg 34: 188-192.
Palmer JH. 1996. Floral initiation and
production in the oil-seed sunflower. – In: Caligari PDS, Hind DJN (eds),
Compositae: biology and utilization. Proceedings of the International
Compositae Conference, Kew, 1994, vol. 2, Royal Botanic Gardens, Kew, pp.
161-178.
Pandey AK, Singh RP, Chopra S. 1978.
Development and structure of seeds and fruits in Compositae-Cichorieae. –
Phytomorphology 28: 198-206.
Pandey AK, Stuessy TF, Mathur R. 2014.
Phytomelanin and systematics of the Heliantheae alliance (Compositae). –
Plant Divers. Evol. 131: 145-165.
Panero JL. 1992. Systematics of
Pappobolus (Asteraceae-Heliantheae). – Syst.
Bot. Monogr. 36: 1-195.
Panero JL. 2005. New combinations and
infrafamilial taxa in the Asteraceae. – Phytologia 87:
1-14.
Panero JL. 2008. Shared molecular signatures
support the inclusion of Catamixis in subfamily Pertyoideae (Asteraceae). – Phytologia 90:
418-424.
Panero J: 2016. Phylogenetic uncertainty and
fossil calibration of Asteraceae
chronograms. – Proc. Natl. Acad. Sci. U.S.A. 113, E411.
http://dx.doi.org/10.1073/pnas.1517649113
Panero JL, Crozier BS. 2016.
Macroevolutionary dynamics in the early diversification of Asteraceae. – Mol. Phylogen. Evol.
99: 116-132.
Panero JL, Funk VA. 2002. Toward a
phylogenetic subfamilial classification for the Compositae (Asteraceae). – Proc. Biol. Soc.
Washington 115: 909-922.
Panero JL, Funk VA. 2007. New infrafamilial
taxa in Asteraceae. – Phytologia
89: 356-360.
Panero JL, Funk VA. 2008. The value of
sampling anomalous taxa in phylogenetic studies: major clades of the Asteraceae revisited. – Mol.
Phylogen. Evol. 47: 757-782.
Panero JL, Funk VA. 2009. New tribes in Asteraceae. – Phytologia 91:
568-570.
Panero JL, Jansen RK. 1997. Chloroplast DNA
restriction site study of Verbesina (Asteraceae: Heliantheae). – Amer.
J. Bot. 84: 382-392.
Panero JL, Schilling EE. 1988. Revision of
Viguiera sect. Maculatae (Asteraceae: Heliantheae). – Syst.
Bot. 13: 371-406.
Panero JL, Schilling EE. 1992. Two new
species of Simsia (Asteraceae: Heliantheae) from
southern Mexico. – Novon 2: 385-388.
Panero JL, Villaseñor JL. 1996a. Tehuana
calzadae (Asteraceae:
Heliantheae) gen. et sp. nov. from the Pacific coast of Oaxaca, Mexico. –
Syst. Bot. 21: 553-557.
Panero JL, Villaseñor JL, 1996b. Novelties
in Asteraceae from southern
Mexico. – Brittonia 48: 79-90.
Panero JL, Francisco-Ortega J, Jansen RK,
Santos-Guerra A. 1999. Molecular evidence for multiple origins of woodiness and
a New World biogeographic connection of the Macaronesian Island endemic
Pericallis (Asteraceae:
Senecioneae). – Proc. Natl. Acad. Sci. U.S.A. 96: 13886-13891.
Panero JL, Jansen RK, Clevinger JA. 1999.
Phylogenetic relationships of subtribe Ecliptinae (Asteraceae: Heliantheae) based on
chloroplast DNA restriction site data. – Amer. J. Bot. 86: 413-427.
Panero JL, Freire SE, Espinar LA, Crozier BS,
Barboza GE, Cantero JJ. 2014. Resolution of deep nodes yields an improved
backbone phylogeny and a new basal lineage to study early evolution of Asteraceae. – Molec. Phylogen.
Evol. 80: 43-53.
Park DS, Potter D. 2015. Why close relatives
make bad neighbours: phylogenetic conservatism in niche preferences and
dispersal disproves Darwin’s naturalization hypothesis in the thistle tribe.
– Mol. Ecol. 12: 3181-3193.
Park J-M, Kovačić S, Liber Z, Eddie WMM,
Schneeweiss GM. 2006. Phylogeny and biogeography of isophyllous species of
Campanula (Campanulaceae) in the
Mediterranean area. – Syst. Bot. 31: 862-880.
Park S-J, Korompai EJ, Francisco-Ortega J,
Santos-Guerra A, Jansen RK. 2001. Phylogenetic relationships of Tolpis
(Asteraceae: Lactuceae) based on
ndhF sequence data. – Plant Syst. Evol. 226: 23-33.
Parker ES, Jones SB. 1975. A systematic study
of the genus Balduina (Compositae, Heliantheae). – Brittonia 27:
355-361.
Parolly G. 2000. Notes on two neglected
Turkish Asyneuma taxa (Campanulaceae). – Willdenowia
30: 67-75.
Parra O, Marticorena C. 1972. Granos de polen
de plantas Chilenas II: Compositae-Mutisieae. – Gayana, Bot. 21: 1-107.
Pasini E, Funk VA, de Souza-Chies TT, Miotto
STS. 2016. New insights into the phylogeny and biogeography of the
Gerbera-complex (Asteraceae: Mutisieae). – Taxon 65:
547-562.
Patel RN. 1973. Wood anatomy of the
dicotyledons indigenous to New Zealand 2. Escalloniaceae.
– New Zealand J. Bot. 11: 421-434.
Patterson R. 1984. Flavonoid uniformity in
diploid species of Hawaiian Scaevola (Goodeniaceae). – Syst. Bot. 9:
263-265.
Pausinger K. 1951. Die Pollengestaltung der
Cichorieae. – Frankenburg XIII. Sonderheft der Carinthia II.
Payne WW. 1964. A re-evaluation of the genus
Ambrosia (Compositae). – J. Arnold Arbor. 45: 401-438.
Payne WW, Skvarla JJ. 1970. Electron
microscope study of Ambrosia pollen. – Grana 10: 89-100.
Payne WW, Raven PH, Kyhos DW. 1964.
Chromosome numbers in Compositae IV. Ambrosia. – Amer. J. Bot. 51:
419-424.
Peacock WJ. 1959. Pollen tetrads in
Leschenaultia. – Proc. Linn. Soc. New South Wales 84: 271-277.
Peacock WJ. 1963. Chromosome numbers and
cytoevolution in the Goodeniaceae. – Proc. Linn. Soc.
New South Wales 88: 8-27.
Peacock WJ, Smith-White S. 1978.
Cytogeography of Brunonia australis Sm. ex R. Br. – Brunonia 1:
31-43.
Peece SJ Jr, Turner BL. 1953. A taxonomic
study of the genus Chamaechaenactis Rydberg (Compositae). – Madroño
12: 97-103.
Peirson JA, Reznicek AA, Semple JC. 2012.
Polyploidy, infraspecific cytotype variation, and speciation in goldenrods: the
cytogeography of Solidago subsect. Humiles (Asteraceae) in North America. –
Taxon 61: 197-210.
Pellicer J, Garcia S, Garnatje T, Hidalgo O,
Korobkov AA, Dariimaa S, Valles J. 2007. Chromosome counts in Asian
Artemisia L. (Asteraceae)
species: from diploids to the first report of the highest polyploid in the
genus. – Bot. J. Linn. Soc. 153: 301-310.
Pellicer J, Garcia E, Canela MÁ, Garnatje T,
Korobkov AA, Twibell JD, Vallès J. 2010. Genome size dynamics in
Artemisia L. (Asteraceae): following the track of
polyploidy. – Plant Biol. 12: 820-830.
Pellicer J, Vallès J, Korobkov AA, Garnatje
T. 2011. Phylogenetic relationships of Artemisia subg.
Dracunculus (Asteraceae)
based on ribosomal and chloroplast DNA sequences. – Taxon 60: 691-704.
Pelser PB, Houchin R. 2004. Taxonomic studies
on Senecio aquaticus (Asteraceae). A recommendation for the
taxonomic status of Aquaticus and Barbareifolius. – Bot. J.
Linn. Soc. 145: 489-498.
Pelser PB, Watson LE. 2009. Introduction to
Asteroideae. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 495-502.
Pelser PB, Gravendeel B, Meijden R van der.
2002. Tackling speciose genera: species composition and phylogenetic position
of Senecio sect. Jacobaea (Asteraceae) based on plastid and
nrDNA sequences. – Amer. J. Bot. 89: 929-939.
Pelser PB, Gravendeel B, Meijden R van der.
2003. Phylogeny reconstruction n the gap between too little and too much
divergence: the closest relatives of Senecio jacobaea (Asteraceae) according to DNA
sequences and AFLPs. – Mol. Phylogen. Evol. 29: 613-628.
Pelser PB, Hof K van den, Gravendeel B,
Meijden R van der. 2004. The systematic value of morphological characters in
Senecio sect. Jacobaea (Asteraceae) as compared to DNA
sequences. – Syst. Bot. 29: 790-805.
Pelser PB, Vos H de, Theuring HC, Beuerle T,
Vrieling K, Hartmann T. 2005. Frequent gain and loss of pyrrolizidine alkaloids
in the evolution of Senecio sect. Jacobaea (Asteraceae). – Phytochemistry 66:
1285-1295.
Pelser PB, Veldkamp J-F, Meijden R van der.
2006. New combinations in Jacobaea Mill. (Asteraceae: Senecioneae). –
Compositae Newslett. 44: 1-11.
Pelser PB, Nordenstam B, Kadereit JW, Watson
LE. 2007. An ITS phylogeny of tribe Senecioneae (Asteraceae) and a new delimitation of
Senecio L. – Taxon 56: 1077-1104.
Pelser PB, Kennedy AH, Tepe EL, Shidler JB,
Nordenstam B, Kadereit Jwe, Watson LE. 2010. Patterns and causes of
incongruence between plastid and nuclear Senecioneae (Asteraceae) phylogenies. – Amer. J.
Bot. 97: 856-873.
Pelser PB, Tepe EJ, Kennedy AH, Watson LE.
2010. The fate of Robinsonia (Asteraceae): sunk in
Senecio, but still monophyletic? – Phytotaxa 5: 31-46.
Pelser PB, Abbott RJ, Comes HP, Milton JJ,
Möller M, Looseley ME, Cron GV, Barcelona JF, Kennedy AH, Watson LE, Barone R,
Hernández F, Kadereit JW. 2012. The genetic ghost of an invasion past:
colonization and extinction revealed by historical hybridization in
Senecio. – Mol. Ecol. 21: 369-387.
Peng C-I, Hsu C-C. 1978. Chromosome numbers
in Taiwan Compositae. – Bot. Bull. Acad. Sin. 19: 53-66.
Peng Y-L, Gao X-F, Peng L. 2013. Pollen
morphology of Youngia and six related genera (Asteraceae: Cichorieae) and its
systematic significance. – Phytotaxa 139: 39-62.
Pereira Coutinho AX. 1996. Palinologia do
género Carduus L. (Compositae) em Portugal. – An. Jard. Bot. Madrid
54: 347-354.
Pereira Coutinho AX, Dinis AM. 2007. A
contribution to the ultrastructural knowledge of the pollen exine in subtribe
Inulinae (Inuleae, Asteraceae).
– Plant Syst. Evol. 269: 159-170.
Pereira Coutinho AX, Dinis AM. 2009. A light,
scanning electron, and transmission electron microscopic study of pollen wall
architecture in the subtribe Gnaphaliinae (Gnaphalieae, Asteraceae). – Plant Syst. Evol.
283: 79-92.
Pereira Coutinho AX, Almeida da Silva R, Sá
da Bandeira D, Ortiz S. 2012. Pollen morphology in tribe Dicomeae Panero and
Funk (Asteraceae). – Plant Syst.
Evol. 298: 1851-1865.
Pereira Coutinho AX, Ortíz S, Valente M,
França R, Soares M. 2014. A contribution to the knowledge of exine
ultrastructure in subtribe Anisopappinae (Athroismeae, Asteraceae). – Willdenowia 44:
431-437.
Perez De Paz J. 1976. Observaciones sobre la
biología y relaciones de Sventenia bupleuroides F. Q. – Bot.
Macaronés. 1: 51-65.
Perrot ME. 1897. Anatomie comparée des
Gentianées aquatiques (Menyanthées Griseb.). – Bull. Soc. Bot. France 44:
340-354.
Persson K. 1974. Biosystematic studies in the
Artemisia maritima complex in Europe. – Opera Bot. 35: 1-188.
Pesacreta TC, Stuessy TF. 1996.
Autofluorescent walls of connective bases in anthers of Barnadesioideae (Asteraceae), and systematic
implications. – Taxon 45: 473-485.
Pesacreta TC, Sullivan VI, DeVore M. 1994.
The connective base and filament of Acicarpha tribuloides (Calyceraceae). – Amer. J. Bot.
81: 753-759.
Peter G. 2004. The genus Isostigma
(Asteraceae, Heliantheae) in
Paraguay, with a key to the species of the genus. – Willdenowia 34:
529-537.
Peter G. 2009. Systematic revision of the
genus Isostigma Less. (Asteraceae, Coreopsideae). –
Candollea 64: 5-30.
Peter G, Katinas L. 2003. A new type of Kranz
anatomy in Asteraceae. – Aust.
J. Bot. 51: 217-226.
Petit DP. 1997. Generic interrelationships of
the Cardueae (Compositae): a cladistic analysis of morphological data. –
Plant Syst. Evol. 207: 173-203.
Petit DP. 1998. Le genre Echinops L.
(Compositae, Cardueae) I. Position phylétique et interprétation de
l’incapitulescence. – Candollea 43: 467-481.
Petit DP, Mathez J, Qaid H. 2000. Phylogeny
of the Cardueae (Asteraceae) based
on analysis of morphological and palynological characters. – Bocconea 13:
41-53.
Petterson JA. 1997. Revision of the genus
Wahlenbergia (Campanulaceae) in New Zealand. –
New Zealand J. Bot. 35: 9-54.
Philipson WR. 1938. Four new species of
Vernonieae collected by Glaziou in Brasil. – Kew Bull. 7: 298-300.
Philipson WR. 1948. Studies in the
development of the inflorescence V. The raceme of Lobelia Dortmanna L.
and other campanulaceous inflorescences. – Ann. Bot., N. S., 12: 147-156.
Philipson WR. 1953. The relationships of the
Compositae, particularly as illustrated by the morphology of the inflorescence
in the Rubiales and the
Campanulatae. – Phytomorphology 3: 391-404.
Philipson WR, Philipson MN. 1973. A
comparison of the embryology of Forstera L. and Donatia J. R.
et G. Forst. – New Zealand J. Bot. 11: 449-460.
Phillips EP. 1917. A revision of the South
African material of the genus Cyphia Berg. – Ann. South Afr. Mus. 9:
449-473.
Phitos D. 1964. Trilokuläre
Campanula-Arten der Ägäis. – Österr. Bot. Zeitschr. 111:
208-230.
Phitos D. 1965. Die quinquelokulären
Campanula-Arten. – Österr. Bot. Zeitschr. 112: 449-498.
Pinkava DJ. 1967. Biosystematic study of
Berlandiera (Compositae). – Brittonia 19: 285-298.
Pippen RW. 1968. Mexican ‘cacalioid’
genera allied to Senecio (Compositae). – Contr. U.S. Nat. Herb. 34:
365-447.
Pittman AB, Bates V. 1989. A new species of
Polymnia (Compositae: Heliantheae) from the Ouchita mountain region of
Arkansas. – Sida 13: 481-486.
Pittoni H. 1974. Behaarung und
Chromosomenzahlen sternhaariger Leontodon-Sippen. – Phyton (Horn)
16: 165-188.
Plovanich AE, Panero JL. 2004. A phylogeny of
the ITS and ETS for Montanoa (Asteraceae: Heliantheae). – Mol.
Phylogen. Evol. 31: 815-821.
Plunkett GT, Bruhl JJ, Telford IRH. 2009. Two
new, sympatric species of Wahlenbergia (Campanulaceae) from the New
England Tableland escarpment, New South Wales, Australia. – Aust. Syst. Bot.
22: 319-331.
Poddubnaja-Arnoldi W. 1931. Ein Versuch der
anwendung der embryologischen Methode bei der Lösung einiger systematischer
Fragen I. Vergleichende embryologisch-zytologische Untersuchungen über die
Gruppe Cynareae, Fam. Compositae. – Beih. Bot. Centralbl., Abt. II, 48:
141-237.
Podlech D. 1965. Revision der europäischen
und nordafrikanischen Vertreter der Subsect. Heterophylla (Wit.) Fed.
der Gattung Campanula L. – Feddes Repert. 71: 50-187.
Podlech D, Damboldt J. 1964. Zytotaxonomische
Beiträge zur Kenntnis der Campanulaceen in Europa. – Ber. Deutsch. Bot. Ges.
76: 360-369.
Polevova SV. 2006. Review of the sporoderm
ultrastructure of members of the Asterales. – Paleontol. J. 40: S656-S663.
Poli F, Sacchetti G, Bruni A. 1995.
Distribution of internal secretory structures in Tagetes patula (Asteraceae). – Nord. J. Bot. 15:
197-205.
Poljakov PP. 1967. Origin and classification
of the Compositae. – Akad. Nauk Kazakh. S.S.R. Inst. Bot., Alma-Ata. [In
Russian]
Pons A, Boulos L. 1972. Révision
systématique du genre Sonchus L. s.l. III. Étude palynologique. –
Bot. Not. 125: 310-319.
Pontiroli A. 1963. Flora Argentina. Calyceraceae. – Rev. Mus. La
Plata, N. S., IX, Bot. 41: 175-214.
Pope GV. 1983. Cypselas and trichomes as a
source of taxonomic characters in the erlangeoid genera. – Kirkia 12:
203-231.
Pope GV. 1984. Two new species in the tribe
Lactuceae (Compositae) from Zambia. – Kew Bull. 39: 167-168.
Pope GV. 1986. Vernonia chloropappa
(Compositae) and related species in tropical Africa. – Kew Bull. 41:
393-397.
Pope GV. 1988. Notes on Vernonia
(Compositae) in the Flora Zambesiaca area. – Kew Bull. 43: 279-289.
Pope GV. 1990. Notes on Vernonieae
(Compositae) in the Flora Zambesiaca area. – Kew Bull. 45: 697-698.
Pope GV. 1991. Notes on Dicoma Cass.
(Compositae). – Kew Bull. 46: 699-709.
Pornpongrungrueng P, Borchsenius F, Englund
M, Anderberg AA, Gustafsson MHG. 2007. Phylogenetic relationships in
Blumea (Asteraceae:
Inuleae) as evidenced by molecular and morphological data. – Plant Syst.
Evol. 269: 223-243.
Pornpongrungrueng P, Borchsenius F,
Gustafsson MHG. 2009. Relationships within Blumea (Inuleae, Asteraceae) and the utility of the
5S-NTS in species-level phylogeny reconstruction. – Taxon 58: 1181-1193.
Pornpongrungrueng P, Gustafsson MHG,
Borchsenius F, Koyama H, Chantaronothai P. 2016. Blumea (Compositae:
Inuleae) in continental Southeast Asia. – Kew Bull. 71: 1 DOI
10.1007/S12225-016-9612-2
Porter CL. 1943. The genus
Amphipappus Torr. and Gray. – Amer. J. Bot. 30: 481-483.
Poulsen VA. 1903. Pentaphragma
ellipticum sp. nov. Et Bidrag til Kundskab om Slægten
Pentaphragma Wall. – Vidensk. Meddel. Dansk Naturh. For. 1903:
319-330.
Powell AM. 1968a. Additional discussions
pertaining to the congeneric status of Perityle and Laphamia
(Compositae). – Sida 3: 270-278.
Powell AM. 1968b. Chromosome numbers in
Perityle and related genera (Compositae-Peritylinae). – Amer. J.
Bot. 55: 820-828.
Powell AM. 1969. Taxonomy of
Perityle section Pappothrix (Compositae-Peritylinae). –
Rhodora 71: 58-93.
Powell AM. 1972. Taxonomy of Amauria
(Compositae-Peritylinae). – Madroño 21: 516-525.
Powell AM. 1972c. Taxonomy of
Pericome (Compositae-Peritylinae). – Southw. Natur. 18: 335-339.
Powell AM. 1973a. Correllia
(Compositae: Peritylinae): a new monotypic genus from southern Chihuahua,
Mexico. – Brittonia 25: 116-118.
Powell AM. 1973b. Taxonomy of
Perityle section Laphamia (Compositae-Helenieae-Peritylinae).
– Sida 5: 61-128.
Powell AM. 1974. Taxonomy of
Perityle section Perityle (Compositae-Peritylinae). –
Rhodora 76: 229-306.
Powell AM. 1978. Systematics of
Flaveria (Flaveriinae-Asteraceae). – Ann. Missouri Bot.
Gard. 65: 590-636.
Powell AM. 1985. Crossing data as generic
criteria in the Asteraceae. –
Taxon 34: 55-60.
Powell AM, Cuatrecasas J. 1970. Chromosome
numbers in Compositae: Colombian and Venezuelan species. – Ann. Missouri Bot.
Gard. 57: 374-379.
Powell AM, King RM. 1969a. Chromosome numbers
in the Compositae: Colombian species. – Amer. J. Bot. 56: 116-121.
Powell AM, King RM. 1969b. Chromosome number
in the Compositae: West Indian species. – Sida 3: 319-320.
Powell AM, Powell SA. 1978. Chromosome
numbers in Asteraceae. –
Madroño 25: 160-169.
Powell AM, Turner BL. 1963. Chromosome
numbers in the Compositae VII. Additional species from the southwestern United
States and Mexico. – Madroño 17: 128-140.
Powell AM, Turner BL. 1974. A generic
conspectus of the subtribe Peritylinae (Asteraceae-Helenieae) and
reassessment of its tribal position. – Amer. J. Bot. 61: 87-93.
Powell AM, Kyhos DW, Raven PH. 1974.
Chromosome numbers in Compositae X. – Amer. J. Bot. 61: 909-913.
Pozner R, Zanotti C, Johnson LA. 2012.
Evolutionary origins of the Asteraceae capitulum: insights from
Calyceraceae. – Amer. J. Bot.
99: 1-13.
Praglowski J, Grafström E. 1980. The pollen
morphology of the tribe Calenduleae with reference to taxonomy. – Bot. Not.
133: 177-188.
Praglowski J, Grafström E. 1985. The genus
Carpodetus (Escalloniaceae):
a pollen morphological enigma. – Grana 24: 11-21.
Prassler M. 1967. Revision der Gattung
Ursinia. – Mitt. Bot. Staatssamml. München 6: 363-478.
Prebble JM, Cupido CN, Meudt HM,
Garnock-Jones PJ. 2011. First phylogenetic and biogeographical study of the
southern bluebells (Wahlenbergia, Campanulaceae). – Mol. Phylogen.
Evol. 59: 636-648.
Prebble JM, Meudt HM, Garnock-Jones PJ. 2012.
An expanded molecular phylogeny of the southern bluebells
(Wahlenbergia, Campanulaceae) from Australia and
New Zealand. – Aust. Syst. Bot. 25: 11-30.
Presti RML, Oppolzer S, Oberprieler C. 2010.
A molecular phylogeny and a revised classification of the Mediterranean genus
Anthemis s.l. (Compositae, Anthemideae) based on three molecular
markers and micromorphological characters. – Taxon 59: 1441-1456.
Pringle JS. 1995. 159B. Menyanthaceae. – In: Harling G,
Andersson L (eds), Flora of Ecuador 53, Nord. J. Bot., Copenhagen, pp.
133-145.
Proksch P. 1985. Vorkommen und biologische
Bedeutung von Benzopyranen (Chromenen) und Benzofuranen in den Asteraceae. – Plant Syst. Evol.
150: 89-100.
Proksch P, Kunze A. 1996. Chemosystematic
evidence from prenylated acetophenones – conclusions at the tribal, inter-
and intrageneric level. – In: Hind DJN Beentje HJ (eds), Compositae:
systematics. Proceedings of the International Compositae Conference, Kew, 1994,
vol. 1, Royal Botanic Gardens, Kew, pp. 295-305.
Proksch P, Rodrigues E. 1983. Chromenes and
benzofurans of the Asteraceae,
their chemistry and biological significance. – Phytochemistry 22:
2335-2348.
Pruski JF. 1991. Compositae of the Guayana
Highland V. The Mutisieae of the lost world of Brazil, Colombia, and Guyana.
– Bol. Mus. Paraense Emilio Goeldi, ser. Bot., 7: 335-392.
Pruski JF. 1997. Proposal to conserve the
name Acanthospermum against Centrospermum (Compositae,
Heliantheae). – Taxon 46: 805-806.
Pruski JF. 1998. Novelties in Calea
(Compositae: Heliantheae) from South America. – Kew Bull. 53: 683-693.
Pruski JF. 2004a. Panphalea
heterophylla (Compositae: Mutisioideae: Nassauvieae), a genus and species
new for the flora of North America. – Sida 21: 1225-1227.
Pruski JF. 2004b. Asteraceae (Compositae. Missouri
Botanical Garden: research. – Missouri Botanical Garden, St. Louis,
Missouri.
Pruski JF. 2014.
Lowryanthusrubens (Compositae: Athroismeae), a new genus and
species from southeastern Madagascar. – Phytoneuron 2014-51: 1-11.
Pruski JF, Hind DJN. 1998. Two new species of
Calea (Compositae: Heliantheae) from Serra Do Grão Mogol and
vicinity, Minas Gerais, Brazil. – Kew Bull. 53: 695-701.
Pullaiah T. 1979. Embryology of
Adenostemma, Elephantopus and Vernonia (Compositae).
– Bot. Not. 132: 51-56.
Pullaiah T. 1983. Studies in the embryology
of Senecioneae (Compositae). – Plant Syst. Evol. 142: 61-70.
Pullaiah T. 1984. Embryology of Compositae.
– Intern. Biosci. Ser. 10. Today and Tomorrow’s Printers and Publ., New
Delhi.
Pusalkar PK, Singh DK. 2005. Proposal to
conserve the name Waldheimia against Allardia (Asteraceae-Anthemideae). – Taxon
54: 553-554.
Pusset J, La-Barre S, Langois N, Hamon J.
1989. Alkaloids of Phelline comosa var. robusta. –
Phytochemistry (Oxford) 28: 1298-1300.
Puttock CF. 1994a. Re-analysis of
Anderberg’s Gnaphalieae data matrix. – Compositae Newsl. 25- 1-14.
Puttock CF. 1994b. Anatomy and morphology of
Cremnothamnus (Asteraceae) a new genus for
Helichrysum thomsonii. – Aust. Syst. Bot. 7: 569-583.
Qaiser M, Lack HW. 1985. The genus
Phagnalon (Asteraceae,
Inuleae) in Arabia. – Willdenowia 18: 3-22.
Qaiser M, Lack HW. 1986. Aliella, a
new genus of Asteraceae (Inuleae)
from Morocco. – Bot. Jahrb. Syst. 106: 487-498.
Quézel P. 1953. Les Campanulacées
d’Afrique du Nord. – Feddes Repert. 56: 1-65.
Queiros M. 1973. Contribucão para o
conhecimento citotaxonômico das Spermatophyta de Portugal II. Compositae. –
Bol. Soc. Brot. 47: 299-314.
Quijano L, Calderon JS, Gomez F, Garduno JT,
Rios T. 1980. Deltoidin A and B, two new germacrolides from Eupatorium
deltoideum. – Phytochemistry 12: 1975-1977.
Quijano L, Calderon JS, Gomez F, Soria IE,
Rios T. 1980. Highly oxygenated flavonoids from Ageratum corymbosum.
– Phytochemistry 19: 2439-2442.
Quijano L, Calderon JS, Gomez F, Vega JL,
Rios T. 1982. Diterpenes from Stevia monardifolia. – Phytochemistry
21: 1369-1371.
Quijano L, Calderon JS, Gomez F, Rios T.
1982a. Four flavonoids from Ageratum strictum. – Phytochemistry 21:
2575-2578.
Quijano L, Calderon JS, Gomez F, Rios T.
1982b. Two polymethoxylated flavonoids from Ageratum houstonianum. –
Phytochemistry 21: 2965-2967.
Raab-Straube E von. 2003. Phylogenetic
relationships in Saussurea (Compositae, Cardueae) sensu lato, inferred
from morphological, ITS and trnL-trnF sequence data, with a
synopsis of Himalaiella gen. nov., Lipschitziella and
Frolovia. – Willdenowia 33: 379-402.
Raab-Straube E von. 2009. Saussurea
luae (Compositae, Cardueae), a new species of snow lotus from China. –
Willdenowia 39: 101-106.
Raamsdonk LWD. 1987. Some aspects of
evolution within the genus Ornithogalum (Liliaceae). –
Evol. Trends Plants 2: 83-84.
Rabakonandrianina E. 1980 [1981].
Infrageneric relationships and the origin of the Hawaiian endemic genus
Lipochaeta (Compositae). – Pacific Sci. 34: 29-39.
Radford IJ, Muller P, Fiffer S, Michael PW.
2000. Genetic relationships between Australian fireweed and South African and
Madagascan populations of Senecio madagascariensis Poir. and closely
related Senecio species. – Aust. Syst. Bot. 13: 409-423.
Radford IJ, Cousens RD, Michael PW. 2004.
Morphological and genetic variation in the Senecio pinnatifolius
complex: are variants worthy of taxonomic recognition? – Aust. Syst. Bot. 17:
29-48.
Raimondo FM, Spadaro V. 2008. A new species
of Centaurea (Asteraceae)
from Sicily. – Bot. J. Linn. Soc. 157: 785-788.
Rajput MTM. 1980. Two new species of
Dampiera (Goodeniaceae)
from Central Australia. – Telopea 2: 57-58.
Rajput MTM, Carolin RC. 1984. Stem structure
and vascularisation in Dampiera (Goodeniaceae). – Proc. Linn. Soc.
New South Wales 107: 479-485.
Rajput MTM, Carolin RC. 1988. The genus
Dampiera (Goodeniaceae): systematic
arrangement, nomenclatural notes and new taxa. – Telopea 3: 183-216.
Rajput MTM, Carolin RC. 1989. Systematic
structure of Dampiera R. Br. (Goodeniaceae), using techniques of
numerical taxonomy. – Pakistan J. Bot. 21: 170-184.
Rajput MTM, Caroin RC. 1992. Phylogenetic
study of Dampiera species by Wagner Tree Algorithm. – Pakistan J.
Bot. 24: 173-181.
Rajput MTM, Carolin RC, Tahir SS. 1985. The
indumentum of the genus Dampiera R. Br. (Goodeniaceae). – Pakistan J. Bot.
17: 181-194.
Ramamonjiarisoa BA. 1980. Comparative anatomy
and systematics of African and Malagasy woody Saxifragaceae
sensu lato. – Ph.D. diss., University of Massachusetts, Amherst,
Massachusetts.
Ramayya N. 1962. Studies on the trichomes of
some Compositae I. General structure. – Bull. Bot. Surv. India 4: 177-188.
Ramos IE La Serna, Mederos MAP. 2008. Pollen
morphology of endemic species of the Gonospermum Less., Lugoa
DC. and Tanacetum L. complex (Asteraceae: Anathemideae) in the
Canary Islands (Spain), and its taxonomical implications. – Grana 47:
247-261.
Randeria AJ. 1960. The composite genus
Blumea, a taxonomic revision. – Blumea 10: 176-317.
Ranjbar M, Negaresh K. 2013. A revision of
Centaurea sect. Phaeopappus (Asteraceae, Cardueae). – Phytotaxa
123: 1-40.
Ranjbar M, Negaresh K, Karamina R, Joharchi
MR. 2015. A synopsis of Klasea sect. Schumeria (Asteraceae, Cardueae) in Iran. –
Novon 24: 186-198.
Rapson LJ. 1953. Vegetative anatomy of
Donatia, Phyllachne, Forstera, and
Oreostylidium and its taxonomic significance. – Trans. Proc. Roy.
Soc. New Zealand 80: 399-402.
Raulings EJ. 2001. Floral morphology,
phylogeny, and pollination ecology in Stylidium section
Stylidium (Stylidiaceae). – Ph.D. diss.,
University of Melbourne, Victoria, Australia.
Raulings EJ, Ladiges PY. 2001. Morphological
variation and speciation in Stylidium graminifolium (Stylidiaceae), description of
S. montanum and reinstatement of S. armeria. – Aust. Syst.
Bot. 14: 901-935.
Rauscher JT. 2002. Molecular phylogenetics of
the Espeletia complex (Asteraceae): evidence from nrDNA ITS
sequences of the closest relatives of an Andean adaptive radiation. – Amer.
J. Bot. 89: 1074-1084.
Raven PH, Kyhos DW. 1961. Chromosome numbers
in Compositae II. Helenieae. – Amer. J. Bot. 48: 842-850.
Raven PH, Solbrig OT, Kyhos DW, Snow R. 1960.
Chromosome numbers in Compositae I. Astereae. – Amer. J. Bot. 47: 124-132.
Rayment T. 1948. Notes on the pollination of
trigger-plants. – Victorian Natur. 65: 118-119.
Raynal A. 1974a. Le genre Nymphoides
(Menyanthaceae) en Afrique et a
Madagascar 1. Morphologie. – Adansonia, sér. II, 14: 227-270.
Raynal A. 1974b. Le genre Nymphoides
(Menyanthaceae) en Afrique et a
Madagascar 2. Taxonomie. – Adansonia, sér. II, 14: 405-458.
Razaq ZA, Vahidy AA. Ali SI. 1994. Chromosome
numbers in Compositae from Pakistan. – Ann. Missouri Bot. Gard. 81:
800-808.
Rechinger KH. 1986. Coussinia:
morphology, taxonomy, distribution and phytogeographical implifications. –
Proc. Roy. Sec. Edinb. 89B: 45-58.
Redonda-Martínez R. 2017. Morfología floral
de la subtribe Leiboldiinae (Vernonieae, Asteraceae). – Brittonia, DOI
10.1007/s12228-017-9487-z
Redonda-Martínez R. 2018. Taxonomic revision
of subtribe Leiboldiinae (Vernonieae: Asteraceae). – Syst. Bot. 43:
344-363.
Reese H. 1989. Die Entwicklung von Perikarp
und Testa bei Calenduleae und Arctotideae (Asteraceae) – ein Beitrag zur
Systematik. – Bot. Jahrb. Syst. 110: 325-419.
Reiche K. 1901. Beiträge zur Systematik der
Calyceraceen. – Engl. Bot. Jahrb. Syst. 29: 107-119.
Reitbrecht F. 1974. Fruchtanatomie und
Systematik der Anthemideae (Asteraceae). – Ph.D. diss.,
Philosophischen Fakultät, Universität Wien, Austria.
Reveal JL. 1997. Early suprageneric names in
Asteraceae. – Compositae
Newslett. 30: 29-45.
Ricardi M. 1959. Un Cyphocarpus
nuevo para Chile. – Bol. Soc. Argent. Bot. 7: 247-250.
Richards AJ. 1972. The karyology of some
Taraxacum species from alpine regions of Europe. – Bot. J. Linn.
Soc. 65: 47-59.
Richards AJ. 1973. The origin of
Taraxacum agamospecies. – Bot. J. Linn. Soc. 66: 189-211.
Richards AJ. 1985. Sectional nomenclature in
Taraxacum (Asteraceae).
– Taxon 34: 633-644.
Richards JH, Dow M, Troxler T. 2010. Modeling
Nymphoides architecture: a morphological analysis of Nymphoides
aquatica (Menyanthaceae).
– Amer. J. Bot. 97: 1761-1771.
Rieseberg LH. 1991. Homoploid reticulate
evolution in Helianthus (Asteraceae): evidence from ribosomal
genes. – Amer. J. Bot. 78: 1218-1237.
Rieseberg LH. 2006. Hybrid speciation in wild
sunflowers. – Ann. Missouri Bot. Gard. 93: 34-48.
Rieseberg LH, Schilling EE. 1985. Floral
flavonoids and ultraviolet patterns in Viguiera (Compositae). –
Amer. J. Bot. 72: 999-1004.
Rieseberg LH, Carter R, Zona S. 1990.
Molecular tests of the hypothesized hybrid origin of two diploid
Helianthus species (Asteraceae). – Evolution 44:
1498-1511.
Rieseberg LH, Beckstrom-Sternberg SM. 1990.
Helianthus annuus ssp. texanus has chloroplast DNA and
nuclear ribosomal RNA genes of Helianthus debilis ssp.
cucumerifolius. – Proc. Natl. Acad. Sci. U.S.A. 87: 593-597.
Rieseberg LH, Beckstrom-Sternberg SM, Liston
A, Arias DM. 1991. Phylogenetic and systematic inferences from chloroplast DNA
and isozyme variation in Helianthus sect. Helianthus (Asteraceae). – Syst. Bot. 16:
50-76.
Rieseberg LH, Linder CR, Seiler GJ. 1995.
Chromosomal and genic barriers to introgression in Helianthus. –
Genetics 141: 1163-1171.
Rieseberg LH, Whitton J, Gardner K. 1999.
Hybrid zones and the genetic architecture of a barrier to gene flow between two
sunflower species. – Genetics 152: 713-727.
Riggins CW, Seigler DS. 2012. The genus
Artemisia (Asteraceae:
Anthemideae) at a continental crossroads: molecular insights into migrations,
disjunctions, and reticulations among Old and New World species from a
Beringian perspective. – Mol. Phylogen. Evol. 64: 471-490.
Rios T, Romo de Vivar A, Romo J. 1967.
Stevin, a new pseudoguaianolide isolated from Stevia rhombifolia. –
Tetrahedron 23: 4265-4269.
Rivera VL, Panero JL, Schilling EE, Crozier
BS, Moraes MD. 2016. Origins and recent radiation of Brazilian Eupatorieae (Asteraceae) in the eastern Cerrado
and Atlantic Forest. – Mol. Phylogen. Evol. 97: 90-100.
Robba L, Carine MA, Russell SJ, Raimondo FM.
2005. The monophyly and evolution of Cynara L. (Asteraceae) sensu lato: evidence from
the Internal Transcribed Spacer region of nrDNA. – Plant Syst. Evol. 253:
53-64.
Roberts RP, Urbatsch LE. 2003. Molecular
phylogeny of Ericameria (Asteraceae, Astereae) based on
nuclear ribosomal 3’ETS and ITS sequence data. – Taxon 52: 209-228.
Roberts RP, Urbatsch LE. 2004. Molecular
phylogeny of Chrysothamnus and related genera (Asteraceae, Astereae) based on
nuclear ribosomal 3’ ETS and ITS sequence data. – Syst. Bot. 29:
199-215.
Robichaux RH. 1985. Tissue elastic properties
of a mesic forest Hawaiian Dubautia species with 13 pairs of
chromosomes. – Pacific Sci. 39: 191-194.
Robichaux RH, Morse S. 1990. Extracellular
polysaccharide and leaf capacitance in a Hawaiian bog species,
Argyroxiphium grayanum (Compositae-Madiinae). – Amer. J. Bot. 77:
134-138.
Robichaux RH, Carr GD, Liebman M, Pearcy RW.
1990. Adaptive radiation of the Hawaiian silversword alliance
(Compositae-Madiinae): ecological, morphological, and physiological diversity.
– Ann. Missouri Bot. Gard. 77: 64-72.
Robins DJ. 1977. Senecioneae – chemical
review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae, Academic Press, London, pp. 831-850.
Robinson BL. 1913a. A generic key to the
Compositae-Eupatorieae. – Contr. Gray Herb. XLII: 429-437.
Robinson BL. 1913b. Revisions of
Alomia, Ageratum and Oxylobus. – Contr. Gray Herb.
XLII: 438-491.
Robinson BL. 1930a. Observations on the genus
Stevia. – Contr. Gray Herb., N. S., 90: 36-58.
Robinson BL. 1930b. The stevias of the
Argentine republic. – Contr. Gray Herb., N. S., 90: 58-79.
Robinson BL. 1930c. The stevias of Paraguay.
– Contr. Gray Herb., N. S., 90: 79-90.
Robinson BL. 1930d. The stevias of North
America. – Contr. Gray Herb., N. S., 90: 90-160.
Robinson BL. 1931a. The stevias of Colombia.
– Contr. Gray Herb., N. S., 96: 28-36.
Robinson BL. 1931b. The stevias of Venezuela.
– Contr. Gray Herb., N. S., 96: 37-43.
Robinson BL. 1931c. The stevias of Ecuador.
– Contr. Gray Herb., N. S., 96: 43-49.
Robinson BL. 1932a. The stevias of Peru. –
Contr. Gray Herb., N. S., 100: 20-36.
Robinson BL. 1932b. The stevias of Bolivia.
– Contr. Gray Herb., N. S., 100: 36-39.
Robinson H. 1974. Studies in the Senecioneae
(Asteraceae) VI. The genus
Arnoglossum. – Phytologia 28: 294-295.
Robinson H. 1975. Studies in the Senecioneae
(Asteraceae) VII. Additions to the
genus Roldana. – Phytologia 32: 331-332.
Robinson H. 1976. Studies in the Liabeae (Asteraceae) III. Notes on the genus
Cacosmia. – Phytologia 34: 46-52.
Robinson H. 1977. An analysis of the
characters and relationships of the tribes Eupatorieae and Vernonieae (Asteraceae). – Syst. Bot. 2:
199-208.
Robinson H. 1978a. Studies in the Senecioneae
(Asteraceae) VIII. A new species
of Psacalium from Mexico. – Phytologia 38: 356-358.
Robinson H. 1978b. Studies in the Senecioneae
(Asteraceae) IX. A new genus,
Dresslerothamnus. – Phytologia 40: 493-494.
Robinson H. 1978c. Studies in the Heliantheae
(Asteraceae) XIV. Validation of
subtribes. – Phytologia 41: 39-44.
Robinson H. 1978d. Studies in the Heliantheae
(Asteraceae) XV. Various new
species and combinations. – Phytologia 41: 33-44.
Robinson H. 1978e. Studies in the Heliantheae
(Asteraceae) XII. Re-establishment
of the genus Smallanthus. – Phytologia 39: 47-53.
Robinson H. 1978f. 190(2).
Compositae-Liabeae. – In: Harling G, Sparre B (eds), Flora of Ecuador,
Swedish Natural Science Research Council, Stockholm, 8: 1-62.
Robinson H. 1979. Two new genera of the
Vernonieae (Asteraceae) from
Brazil, Heterocypsela and Pseudostifftia. – Phytologia 44:
442-450.
Robinson H. 1981a. A revision of the tribal
and subtribal limits of the Heliantheae (Asteraceae). – Smithsonian Contr.
Bot. 51: 1-102.
Robinson H. 1981b. Episcothamnus and
Bishopalea, two new genera of Vernonieae (Asteraceae) from Brasil, and the
resurrection of Sipolisia. – Phytologia 48: 209-217.
Robinson H. 1983a. Studies in the Liabeae (Asteraceae) XVI. New taxa from Peru.
– Phytologia 54: 62-65.
Robinson H. 1983b. A generic review of the
tribe Liabeae (Asteraceae). –
Smithsonian Contr. Bot: 54: 1-69.
Robinson H. 1984. Style rotation in the Asteraceae. – Taxon 33: 400-404.
Robinson H. 1987a. Some suggestions regarding
the significance of chloroplast DNA variation in the Asteraceae. – Phytologia 65:
316-324.
Robinson H. 1987b. Studies in the
Lepidaploa Complex (Vernonieae: Asteraceae) II – a new genus,
Echinocoryne. – Proc. Biol. Soc. Washington 100: 584-589.
Robinson H. 1988a. A new species of
Trichocline from northern Peru. – Phytologia 65: 47-49.
Robinson H. 1988b. Studies in the
Lepidaploa complex (Vernonieae: Asteraceae) IV – the new genus,
Lessingianthus. – Proc. Biol. Soc. Washington 101: 929-951.
Robinson H. 1988c. Studies in the
Lepidaploa complex (Vernonieae: Asteraceae) V – the new genus
Chrysolaena. – Proc. Biol. Soc. Washington 101: 952-958.
Robinson H. 1988d. Studies in the
Lepidaploa complex (Vernonieae: Asteraceae) VI – a new genus,
Aynia. – Proc. Biol. Soc. Washington 101: 959-965.
Robinson H. 1989. A revision of the genus
Dresslerothamnus (Asteraceae: Senecioneae). – Syst.
Bot. 14: 380-388.
Robinson H. 1990a. Notes on
Sinclairia and Liabellum in Mesoamerica (Liabeae:Asteraceae). – Phytologia 69:
57-60.
Robinson H. 1990b. Notes on Ageratum
in Mesoamerica (Eupatorieae: Asteraceae). – Phytologia 69:
93-104.
Robinson H. 1990c. A redelimitation of
Microliabum Cabrera (Asteraceae: Liabeae). – Syst. Bot.
15: 736-744.
Robinson H. 1990d. Studies in the
Lepidaploa Complex (Vernonieae: Asteraceae) VII – the genus
Lepidaploa. – Proc. Biol. Soc. Washinton 103: 464-498.
Robinson H. 1991a. A review of the genus
Macvaughiella (Eupatorieae: Asteraceae) with two new species. –
Syst. Bot. 16: 639-643.
Robinson H. 1991b. Two new species of
Stifftia with notes on relationships of the genus (Asteraceae: Mutisieae). – Syst.
Bot. 16: 685-692.
Robinson H. 1992. Observations on the unique
form of sweeping hairs on the styles of the Eremothamneae (Asteraceae). – Taxon 41:
199-200.
Robinson H. 1993. three new genera of
Vernonieae from South America, Dasyandantha, Dasyanthina, and
Quechualia (Asteraceae).
– Proc. Biol. Soc. Washington 106: 775-785.
Robinson H. 1994a. Notes on the tribes
Eremothamneae, Gundelieae, and Moquinieae, with comparisons of their pollen.
– Taxon 43: 33-44.
Robinson H. 1994b. Cololobus,
Pseudopiptocarpha and Trepadonia, 3 new genera from South
America (Vernonieae-Asteraceae).
– Proc. Biol. Soc. Washington 107: 557-568.
Robinson H. 1996a. The status of generic and
subtribal revisions in the Vernonieae. – In: Hind DJN, Beentje HJ (eds),
Compositae: systematics. Proceedings of the International Compositae
Conference, Kew, 1994, vol. 1, Royal Botanic Gardens, Kew, pp. 511-529.
Robinson H. 1996b. Recent studies in the
Heliantheae and Eupatorieae. – In: Hind DJN, Beentje HJ (eds), Compositae:
systematics. Proceedings of the International Compositae Conference, Kew, 1994,
vol. 1, Royal Botanic Gardens, Kew, pp. 627-653.
Robinson H. 1997. New species of
Aphanactis in Ecuador and Bolivia and new combinations in
Selloa (Heliantheae: Asteraceae). – Brittonia 49:
71-78.
Robinson H. 1998. Two new species of
Lepidaploa (Vernonieae: Asteraceae). – Phytologia 84:
40-42.
Robinson H. 1999a. Revisions of paleotropical
Vernonieae (Asteraceae). – Proc.
Biol. Soc. Washington 112: 220-247.
Robinson H. 1999b. Generic and subtribal
classification of American Vernonieae. – Smithsonian Contr. Bot. 89: i-iv,
1-116.
Robinson H. 2002. Holoschkuhria, a
new genus of the Hymenopappinae (Helenieae) from Peru. – Compositae Newslett.
38: 47-51.
Robinson H. 2005. Parapolydora (Asteraceae), a new genus of
Vernonieae from South Africa. – Phytologia 87: 75-79.
Robinson H. 2008. 190(3).
Compositae-Eupatorieae. – In: Harling G, Persson C (eds), Flora of Ecuador
83, Department of Plant and Environmental Sciences, Göteborg University, pp.
1-347.
Robinson H. 2009a. An introduction to
micro-characters of Compositae. – In: Funk VA, Susanna A, Stuessy TF, Bayer
RJ (eds), Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 89-100.
Robinson H. 2009b. Moquinieae. – In: Funk
VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and
biogeography of Compositae, International Association for Plant Taxonomists,
Wien, pp. 477-481.
Robinson H. 2011. A new monotypic genus,
Ananthura, from Tropical Africa (Asteraceae, Vernonieae). – Novon
21: 251-255.
Robinson H. 2015a. Notes on the genus
Chionolaena in Colombia with a new species Chionolaena
barclayae (Asteraceae,
Gnaphalieae). – PhytoKeys 46: 67-71.
Robinson H. 2015b. The genus
Fleischmannia in Argentina, Bolivia, Brazil and Paraguay (Eupatorieae,
Asteraceae). – PhytoKeys 57:
61-92.
Robinson H, Brettell RD. 1972. Tribal
revisions in the Asteraceae I. The
relationship of Geissolepis. – Phytologia 24: 299-301.
Robinson H, Brettell RD. 1973a. Tribal
revisions in the Asteraceae II.
The relationship of Trichospira. – Phytologia 25: 259-261.
Robinson H, Brettell RD. 1973b. Tribal
revisions in the Asteraceae III. A
new tribe, Liabeae. – Phytologia 25: 404-407.
Robinson H, Brettell RD. 1973c. Tribal
revisions in the Asteraceae V. The
relationship of Rigiopappus. – Phytologia 26: 69-70.
Robinson H, Brettell RD. 1973d. Tribal
revisions in the Asteraceae VI.
The relationship of Eriachaenium. – Phytologia 26: 71-72.
Robinson H, Brettell RD. 1973e. Tribal
revisions in the Asteraceae VII.
The relationships of Isoetopsis. – Phytologia 26: 73-75.
Robinson H, Brettell RD. 1973f. Tribal
revisions in the Asteraceae VIII.
A new tribe, Ursinieae. – Phytologia 26: 76-86.
Robinson H, Brettell RD. 1973g. Tribal
revisions in the Asteraceae IX.
The relationship of Ischnea. – Phytologia 26: 153-158.
Robinson H, Brettell RD. 1973h. Tribal
revisions in the Asteraceae X. The
relationship of Plagiocheilus. – Phytologia 26: 159-162.
Robinson H, Brettell RD. 1973i. Tribal
revisions in the Asteraceae XI. A
new tribe, Eremothamneae. – Phytologia 26: 163-166.
Robinson H, Brettell RD. 1973j Studies in the
Senecioneae (Asteraceae) I. A new
genus, Pittocaulon. – Phytologia 26: 451-453.
Robinson H, Brettell RD. 1973k. Studies in
the Senecioneae (Asteraceae) II. A
new genus, Nelsonianthus. – Phytologia 27: 53-54.
Robinson H, Brettell RD. 1973l. Studies in
the Senecioneae (Asteraceae) III.
The genus Psacalium. – Phytologia 27: 254-264.
Robinson H, Brettell RD. 1973m. Studies in
the Senecioneae (Asteraceae) IV.
The genera Mesadenia, Syneilesis, Miricacalia,
Koyamacalia, and Roldana. – Phytologia 27: 265-276.
Robinson H, Brettell RD. 1974a. Studies in
the Senecioneae (Asteraceae) V.
The genera Psacaliopsis, Barkleyanthus,
Telanthophora and Roldana. – Phytologia 27: 402-439.
Robinson H., Brettell RD. 1974b. Studies in
the Liabeae (Asteraceae) II.
Preliminary survey of the genera. – Phytologia 28: 43-63.
Robinson H, Brouillet L. 1994. Notes on the
tribes Eremothamneae, Gundelieae, and Moquinieae, with comparisons of their
pollen. – Taxon 43: 33-44.
Robinson H, Cuatrecasas J. 1973. The generic
limits of Pluchea and Tessaria. – Phytologia 27:
277-285.
Robinson H, Cuatrecasas J. 1994.
Jessea and Talamancalia, two new genera of the Senecioneae
(Asteraceae) from Costa Rica and
Panama. – Novon 4: 48-52.
Robinson H, Funk VA. 1987. A phylogenetic
analysis of Leiboldia, Lepidonia, and a new genus
Stramentopappus (Vernonieae: Asteraceae). – Bot. Jahrb. Syst.
108: 213-228.
Robinson H, Funk VA. 2000. Proposal to
conserve the name Wulffia against Tilesia (Asteraceae). – Taxon 49:
569-570.
Robinson H, Funk VA. 2009. Eremothamneae. –
In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and
biogeography of Compositae, International Association for Plant Taxonomists,
Wien, pp. 411-416.
Robinson H, Funk VA. 2011a. A new genus,
Nothovernonia, from tropical Africa (Asteraceae or Compositae,
Vernonieae). – PhytoKeys 3: 21-34.
Robinson H, Funk VA. 2011b. Stephanbeckia
plumosa (Liabeae: Compositae): a new genus and species from southern
Bolivia. – Brittonia 63: 75-82.
Robinson H, Funk VA. 2012.
Cuatrecasanthus (Vernonieae, Compositae): a revision of a
north-central Andean genus. – PhytoKeys 14: 23-41.
Robinson H, Funk V. 2014. Dysaster
cajamarcensis, a new shrubby genus and species of Astereae (Asteraceae) from Peru. – PhytoKeys
36: 35-40.
Robinson H, Kahn B. 1986. Trinervate leaves,
yellow flowers, tailed anthers, and pollen variation in Distephanus
Cassini (Vernonieae: Asteraceae).
– Proc. Biol. Soc. Washington 99: 493-501.
Robinson H, King RM. 1977a. Eupatorieae –
systematic review. – In: Heywood VH, Harborne JB, Turner BL (eds), The
biology and chemistry of the Compositae, Academic Press, London, pp.
437-485.
Robinson H, King RM. 1977b. Comments on the
generic concepts in the Eupatorieae. – Taxon 34: 11-16.
Robinson H, Marticorena C. 1986. A
palynological study of the Liabeae (Asteraceae). – Smithsonian Contr.
Bot. 64: 1-50.
Robinson H, Moore AJ. 2004. New species and
new combinations in Rhysolepis (Asteraceae: Heliantheae). – Proc.
Biol. Soc. Washington 117: 423-446.
Robinson H, Panero JL. 1994. Idiopappus
quitensis gen. et sp. nov. from Ecuador (Asteraceae: Heliantheae). – Syst.
Bot. 19: 359-362.
Robinson H, Skvarla JJ. 2006. Studies on the
Gymnantheminae (Vernonieae: Asteraceae) – restoration of the
genus Monosis. – Proc. Biol. Soc. Washington 119: 600-607.
Robinson H, Skvarla JJ. 2007. Studies on the
Gymnantheminae (Vernonieae: Asteraceae) II – a new genus,
Decaneuropsis, from China, India, and southeast Asia. – Proc. Biol.
Soc. Washington 120: 359-366.
Robinson H, Skvarla JJ. 2009a. Studies on the
Paleotropical Veroninieae (Asteraceae): additions to the genus
Acilepis from southern Asia. – Proc. Biol. Soc. Washington 122:
131-145.
Robinson H, Skvarla JJ. 2009b. A new genus,
Khasianthus, from India, Myanmar, and China (Vernonieae: Asteraceae). – Proc. Biol. Soc.
Washington 122: 146-149.
Robinson H, Skvarla JJ. 2009c. A new genus,
Uniyala, from peninsular India and Sri Lanka (Vernonieae: Asteraceae). – Proc. Biol. Soc.
Washington 122: 150-154.
Robinson H, Skvarla JJ. 2010a. A new genus,
Okia, from northern Myanmar (Vernonieae, Asteraceae). – Proc. Biol. Soc.
Washington 123: 87-91.
Robinson H, Skvarla JJ. 2010b. The
restoration of the genus Vernonella Sond. (Vernonieae: Asteraceae). – Proc. Biol. Soc.
Washington 123: 181-192.
Robinson H, Skvarla JJ. 2010c. Genera of the
Vernonieae (Asteraceae) of China
with a study of their pollen. – Taiwania 55: 254-272.
Robinson H, Skvarla JJ. 2011. A new monotypic
genus, Ananthura, from tropical Africa (Asteraceae, Vernonieae). – Novon
21: 251-255.
Robinson H, Skvarla JJ. 2013.
Lettowia, a new genus of Vernonieae from East Africa (Asteraceae). – PhytoKeys 25:
47-53.
Robinson H, Skvarla JJ. 2010. A new genus,
Okia, from northern Myanmar (Vernonieae, Asteraceae). – Proc. Biol. Soc.
Washington 123: 87-91.
Robinson H, Skvarla JJ. 2014. Pantoporate
pollen in the Asteraceae
(Vernonieae). – PhytoKeys 38: 1-13.
Robinson H, Yankowski S. 2016. The taxonomic
significance of ducts in the corolla lobes of Vernonia (Vernonieae: Asteraceae). – PhytoKeys 58:
1-7.
Robinson H, Bohlmann F, King RM. 1980.
Chemosystematic notes on the Asteraceae III. Natural subdivisions
of the Vernonieae. – Phytologia 46: 421-436.
Robinson H, Powell AM, King RM, Weedin JF.
1981. Chromosome numbers in Compositae XII. Heliantheae. – Smithsonian Contr.
Bot. 52: 1-28.
Robinson H, Powell AM, King RM, Weedin JF.
1985. Chromosome numbers in Compositae XI. Liabeae. – Ann. Missouri Bot.
Gard. 72: 469-479.
Robinson H, Powell AM, Carr GD, King RM,
Weedin JF. 1989. Chromosome numbers in Compositae XVI. Eupatorieae II. – Ann.
Missouri Bot. Gard. 76: 1004-1011.
Robinson H, Carr GD, King RM, Powell AM.
1997. Chromosome numbers in Compositae XVII: Senecioneae III. – Ann. Missouri
Bot. Gard. 8: 893-906.
Robinson H & collaborators. 2006a.
190(6). Compositae-Heliantheae. Part I: Introduction, genera A-L. – In:
Harling G, Andersson L (†) (eds), Flora of Ecuador 77(1), Botanical
Institute, Göteborg University, pp. 1-230.
Robinson H & collaborators. 2006b.
190(6). Compositae-Heliantheae. Part II: genera M-Z. – In: Harling G,
Andersson L (†) (eds), Flora of Ecuador 77(2), Botanical Institute, Göteborg
University, pp. 1-233.
Robinson H, Keeley SC, Skvarla JJ, Chan R.
2008. Studies on the Gymnantheminae (Vernonieae: Asteraceae) III – restoration of
the genus Strobocalyx and the new genus Tarlmounia. – Proc.
Biol. Soc. Washington 121: 19-33.
Robinson H, Schilling E, Panero JL. 2009.
Eupatorieae. – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 731-744.
Robinson H, Bunwong S, Chantaranothai P.
2010. A new genus, Kurziella from Thailand (Vernonieae: Asteraceae). – Proc. Biol. Soc.
Washington 123: 174-178.
Robinson H, Keeley SC, Skvarla JJ, Chan R.
2014. Two new genera, Hoffmannanthus and Jeffreycia, mostly
from East Africa (Erlangeinae, Vernonieae, Asteraceae). – PhytoKeys 39:
49-64.
Robinson H, Skvarla J, Funk VA. 2016.
Vernonieae (Asteraceae) of
southern Africa: a generic disposition of the species and a study of their
pollen. – PhytoKeys 60: 49-126.
Rock HFL. 1957. A revision of the vernal
species of Helenium (Compositae). – Rhodora 59: 101-116, 128-158,
168-178, 203-216.
Rodrígues C, Ormond WT, Bezerra Pinheiro MC.
1977. Contribução a citologia de Acicarpha spathulata R. Brown (Calyceraceae). – Bol. Mus. Nac.
Rio de Janeiro, n.s., 45: 1-6.
Roessler H. 1959. Revision der
Arctotideae-Gorteriinae (Compositae). – Mitt. Bot. Staatssamml. München 3:
71-500.
Roessler H. 1973. Nachträge zur Bearbeitung
der Arctotideae-Gorteriinae (Compositae). – Mitt. Bot. Staatssamml. München
11: 91-99.
Rollins RC. 1950. The guayule rubber plant
and its relatives. – Contr. Gray Herb. Harvard Univ. 172: 1-72.
Romaschenko K, Ertugrul K, Susanna A,
Garcia-Jacas N, Uysal T, Arslan E. 2004. New chromosome counts in the
Centaurea jacea group (Asteraceae, Cardueae) and some
related taxa. – Bot. J. Linn. Soc. 145: 345-352.
Rommel A. 1977. Die Gattung Amellus
L. (Asteraceae-Astereae).
Systematischer Teil. – Mitt. Bot. Staatssamml. München 13: 579-728.
Rommel A. 1979. Die Gattung Amellus
L. (Asteraceae-Astereae).
Allgemeiner Teil. – Mitt. Bot. Staatssamml. München 15: 243-328.
Romo J, Rios T, Quijano L. 1968. Ligustrin, a
guaioanolide isolated from Eupatorium ligustrinum. – Tetrahedron 24:
6087-6091.
Roque N, Funk VA. 2013. Morphological
characters add support for some members of the basal grade of Asteraceae. – Bot. J. Linn. Soc.
171: 568-586.
Roque N, Nakajima JN. 2001. Two new species
of Richterago Kuntze emend. Roque (Compositae, Mutisieae) from Minas
Gerais and Goias, Brazil. – Kew Bull. 56: 697-703.
Roque N, Pirani JR. 2001. Reinstatement of
the name Richterago Kuntze and recircumscription of the genus to
include species formerly treated as Actinoseris (Endl.) Cabrera
(Compositae, Mutisieae). – Taxon 50: 1155-1160.
Roque N, Pirani JR. 2014. Taxonomic revision
of Richterago (Asteraceae, Gochnatieae). – Syst.
Bot. 39: 997-1026.
Roque N, Santana FA. 2014. A new species for
a monotypic genus: Anteremanthus (Asteraceae: Vernonieae). – Syst.
Bot. 39: 656-661.
Roque N, Silvestre-Capelato M. 2001. Generic
delimitation of Gochnatia, Richterago and
Ianthopappus (Compositae-Mutisieae) based on pollen morphology. –
Grana 40: 197-204.
Roque N, Gonçalves JM, Dematteis M. 2008. A
new species of the Brazilian genus Chresta (Asteraceae, Vernonieae) from Bahia.
– Bot. J. Linn. Soc. 157: 587-590.
Roque N, Bautista HP, Mota AC da. 2012.
Taxonomic revision of Trichogonia (Eupatorieae, Asteraceae): a South American genus.
– Syst. Bot. 37: 525-553.
Roquet C, Sáez L, Aldasoro JJ, Susanna A,
Alarcón ML, Garcia-Jacas N. 2008. Natural delineation, molecular phylogeny and
floral evolution in Campanula. – Syst. Bot. 33: 203-217.
Roquet C, Sanmartin I, García-Jacas N, Saez
L, Susanna A, Wikström N, Aldasoro JJ. 2009. Reconstructing the history of Campanulaceae with a Bayesian
approach to molecular dating and dispersal-vicariance analyses. – Mol.
Phylogen. Evol. 52: 575-587.
Rosén W. 1932. Zur Embryologie der
Campanulaceen und Lobeliaceen. – Acta Horti Gothob. 7: 31-42.
Rosén W. 1935. Beiträge zur Embryologie der
Stylidiaceen. – Bot. Not. 1935: 273-278.
Rosén W. 1938. Beiträge zur Kenntnis der
Embryologie der Goodeniaceen. – Acta Horti Gothob. 12: 1-10.
Rosén W. 1946. Further notes on the
embryology of the Goodeniaceae.
– Acta Horti Gothob. 16: 235-249.
Rosén W. 1949. Endosperm development in Campanulaceae and closely related
families. – Bot. Not. 1949: 137-147.
Rosenberg O. 1906. Über die Embryobildung in
der Gattung Hieracium. – Ber. Deutsch. Bot. Ges. 24: 157-161.
Rosenberg O. 1908. Cytological studies on the
apogamy in Hieracium. – Bot. Tidsskr. 28: 143-170.
Rosselli S, Bruno M, Maggio A, Raccuglia RA,
Bancheva S, Senatore F, Formisano C. 2009. Essential oils from the aerial parts
of Centaurea cuneifolia Sibth. & Sm. and C. euxina
Velen., two species growing wild in Bulgaria. – Biochem. Syst. Ecol. 37:
426-431.
Rousi A. 1973. Studies on the cytotaxonomy
and mode of reproduction of Leontodon (Compositae). – Ann. Bot.
Fenn. 10: 201-215.
Rousi A, Huttunen H, Hyrkas-Lyytikainen K.
1985. Chromosomes and reproductive behaviour of Finnish Taraxacum
agamospecies. – Nord. J. Bot. 5: 127-141.
Routsi E. 1993. Biosystematic study of the
section Acrocentron (Cass.) DC. of the genus Centaurea L. in
Greece. – Thesis, Academic Press, University of Patras, Greece.
Routsi E, Georgiadis T. 1994. Contribution to
the systematics of the genus Centaurea section Acrocentron
(Asteraceae) in Greece. – Nord.
J. Bot. 14: 369-378.
Roux J, Boulos L. 1972. Révision
systématique du genre Sonchus L. s.l. II. Étude caryologique. –
Bot. Not. 125: 306-309.
Rowley G. 1965. Succulent Compositae 2.
Chromosome numbers. – Natl. Cact. Succ. J. 20: 47-49.
Rowley JR, Dahl AO. 1977. Pollen development
in Artemisia vulgaris with special reference to glycocalyx material.
– Pollen Spores 19: 169-284.
Royen P van. 1973. Two superfluous genera in
the New Guinean flora. – Bot. Not. 126: 417-425.
Rozefelds AC. 2001. The species of
Scaevola (Goodeniaceae)
in Tasmania. – Telopea 9: 345-352.
Rozenblum E, Waisman CE, Hunziker JH. 1985.
Estudios cariológicos en Compositae II. – Darwiniana 26: 15-25.
Ruas CF, Ruas PM, Matzenbacher NI, Ross G,
Bernini C, Vanzela ALL. 1995. Cytogenetic studies of some Hypochoeris
species (Compositae) from Brazil. – Amer. J. Bot. 82: 369-375.
Ruas PM, Vanzela ALL, Vieira AOS, Bernini C,
Ruas CR. 2001. Karyotype studies in Brazilian species of Lobelia L.,
subgenus Tupa (Campanulaceae). – Rev. Bras.
Bot. 24: 249-254.
Ruffin J. 1974. A taxonomic reevaluation of
the genera Amphiachyris, Amphipappus, Greenella,
Guitierrezia, Gymnosperma, Thurovia and
Xanthocephalum (Compositae). – Sida 5: 301-333.
Ruffin J. 1977. Palynological survey of the
genera Amphiachyris, Amphipappus, Greenella,
Gutierrezia, Gymnosperma and Xanthocephalum. –
Contr. Gray Herb. 207: 117-131.
Ruiters AK, Tilney PM, Van Wyk B-E, Magee AR.
2016. Taxonomy of the genus Phymaspermum (Asteraceae, Anthemideae). – Syst.
Bot. 41: 430-456.
Ruiz de Ciolfi EN. 1976. Legenere
McVaugh (Campanulaceae), nueva
cita para la Argentina. – Bol. Soc. Argentina Bot. 17: 176-178.
Runemark H. 1967. Studies in the Aegean flora
XII. Cytologic and morphologic investigations in Centaurea. – Bot.
Not. 120: 161-176.
Rustan ØH. 1981. Infraspecific variation in
Argyranthemum pinnatifidum (Lowe) Lowe. – Bocagiana 55: 2-18.
Rydberg PA. 1927. Carduaceae. Liabeae,
Neurolaeneae, Senecioneae (pars). – North American Flora 34, New York, pp.
289-360.
Ryding O, Bremer K. 1992. Phylogeny,
distribution, and classification of the Coreopsideae (Asteraceae). – Syst. Bot. 17:
649-659.
Saad SI. 1961. Pollen morphology in the genus
Sonchus. – Pollen Spores 3: 247-260.
Saar DE, Sørensen PD, Hjerting JP. 2002.
Dahlia spectabilis (Asteraceae, Coreopsideae), a new
species from San Luis Potosi, Mexico. – Brittonia 54: 116-119.
Saar DE, Polans NO, Sørensen PD. 2003. A
phylogenetic analysis of the genus Dahlia (Asteraceae) based on internal and
external transcribed spacer regions of nuclear ribosomal DNA. – Syst. Bot.
28: 627-639.
Saavedra MM, Guimarães EF, Loeuille B,
Forzza RC. 2018. Taxonomic revision of Dasyphyllum sect.
Macrocephala (Asteraceae:
Barnadesioideae). – Syst. Bot. 43: 297-315.
Sáenz AA. 1981. Anatomía y morfología de
frutos de Heliantheae. – Darwiniana 23: 37-117.
Saez FA. 1949. Estudio citologico comparativo
de algunas especies del género Hypochoeris (Compositae) de la America
del sur. – Lilloa 19: 97-104.
Saez L, Aldasoro JJ. 2003. A taxonomic
revision of Campanula L. subgenus Sicyocodon (Feer) Damboldt
and subgenus Megalocalyx Damboldt (Campanulaceae). – Bot. J. Linn.
Soc. 141: 215-241.
Sahin A, Kiran Y, Arabaci T, Turkoglu I.
2006. Karyological notes on eight species of Achillea L. (Asteraceae, Santolinoideae) from
Turkey. – Bot. J. Linn. Soc. 151: 573-580.
Saklani A, Rao RR, Chaudhary LB. 2000.
SEM-characterisation of achene morphology towards the taxonomy of Indian
species of Saussurea DC. (Asteraceae). – Rheedea 10: 1-18.
Sales FI, Hedge C, Eddie WM, Preston J,
Moeller M. 2002. Jasione L. Taxonomy and phylogeny. – Turkish J.
Bot. 28: 253-259.
Salgado-Labouriau ML. 1982a. On cavities in
spines of Compositae pollen. – Grana 21: 97-102.
Salgado-Labouriau ML. 1982b. Pollen
morphology of the Compositae of the Northern Andes. – Pollen Spores 24:
397-452.
Salmon M, Ortega A, Diaz E. 1975. Structure
and stereochemistry of a new germacrane sesquiterpene lactone. – Rev.
Latinoamer. Quim. 6: 45-48.
Salmon M, Diaz E, Ortega A. 1977.
Epoxilactonas de Stevia serrata. – Rev. Latinoamer. Quim. 8:
172-175.
Salmon M, Ortega A, Garcia de la Mora G,
Angeles E. 1983. A diterpenic acid from Stevia lucida. –
Phytochemistry 22: 1512-1523.
Samuel R, Stuessy TF, Tremetsberger K, Baeza
CM, Šiljak-Yakovlev S. 2003. Phylogenetic relationships among species of
Hypochaeris (Asteraceae,
Cichorieae) based on ITS, plastid trnL intron, trnL-F spacer,
and matK sequences. – Amer. J. Bot. 90: 496-507.
Samuel R, Gutermann W, Stuessy TF, Ruas CF,
Lack H-W, Tremetsberger K, Talavera S, Hermanowski B, Ehrendorfer F. 2006.
Molecular phylogenetics reveals Leontodon (Asteraceae, Cichorieae) to be
diphyletic. – Amer. J. Bot. 93: 1193-1205.
Sánchez-Jiménez I, Lazkov GA, Hidalgo O,
Garnatje T. 2010. Molecular systematics of Echinops L. (Asteraceae, Cynareae): a phylogeny
based on ITS and trnL-trnF sequences with emphasis on
sectional delimitation. – Taxon 59: 698-708.
Sancho G. 1999. Novedades taxonómicas en
Gochnatia (Asteraceae,
Mutisieae). – Novon 9: 557-561.
Sancho G. 2000. Revisión y filogenia de la
sección Moquiniastrum Cabrera del género Gochnatia Kunth
(Asteraceae, Mutisieae). –
Fontqueria 54: 61-122.
Sancho G. 2004. Phylogenetic relationships in
the genus Onoseris (Asteraceae, Mutisieae) inferred from
morphology. – Syst. Bot. 29: 432-447.
Sancho G. 2012. Exostigma, a new
genus of Astereae (Compositae) from southern South America. – Syst. Bot. 37:
516-524.
Sancho G, Freire SE. 2009. Gochnatieae
(Gochnatioideae) and Hyalideae (Wunderlichioideae p.p.). – In: Funk VA,
Susanna A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and biogeography
of Compositae, International Association for Plant Taxonomists, Wien, pp.
249-260.
Sancho G, Karaman-Castro V. 2008. A
phylogenetic study in American Podocominae (Asteraceae: Astereae) based on
morphological and molecular data. – Syst. Bot. 33: 762-775.
Sancho G, Katinas L. 2002. Are the trichomes
in corollas of Mutisieae (Asteraceae) really twin hairs? –
Bot. J. Linn. Soc. 140: 427-433.
Sancho G, Otegui M. 2000. Secretory tissues
in florets of Gochnatia polymorpha (Asteraceae, Mutisieae). – Evolutive
considerations. – Phytomorphology 50: 172-179.
Sancho G, Freire SE, Katinas L, Tellería MC.
2005. A new combination and a new species of Andean Mutisieae (Asteraceae). – Taxon 54: 85-90.
Sancho G, Bonifacino JM, Pruski JF. 2006.
Revision of Microgyne (Asteraceae: Astereae), the correct
name for Microgynella. – Syst. Bot. 31: 851-880.
Sancho G, Funk VA, Roque N. 2013.
Moquiniastrum (Gochnatieae, Asteraceae): disentangling the
paraphyletic Gochnatia. – Phytotaxa 147: 26-34.
Sancho G, Katinas L, Plos A. 2014. Is
morphology supporting a monophyletic Proustia Lag. (Nassauvieae, Asteraceae)? – Plant Syst. Evol.
300: 2265-2276.
Sancho G, Lange PJ de, Donato M, Barkla J,
Wagstaff SJ. 2014. Late Cenozoic diversification of the austral genus
Lagenophora (Astereae, Asteraceae). – Bot. J. Linn. Soc.
177: 78-95.
Sancho G, Katinas L, Barreto JNV,
Moreira-Muñoz A, Luebert F. 2018. Phylogenetic relationships and generic
reassessment of Proustia and allies (Compositae: Nassauvieae). –
Taxon 67: 113-129.
Sanders RW. 1977. Taxonomy of
Rumfordia (Asteraceae).
– Syst. Bot. 2: 302-316.
Sanders RW, Stuessy TF, Marticorena C, Silva
O M. 1987. Phytogeography and evolution of Dendroseris and
Robinsonia, tree-Compositae of the Juan Fernandez Islands. – Opera
Bot. 92: 195-215.
Sandwith NY. 1956. Contributions to the flora
of tropical America LXI. Notes on Philoglossa. – Kew Bull. 1956:
289-293.
Sang T, Crawford DJ, Kim S-C, Stuessy TF.
1994. Radiation of the endemic genus Dendroseris (Asteraceae) on the Juan Fernandez
Islands: evidence from sequences of the ITS regions of nuclear ribosomal DNA.
– Amer. J. Bot. 81: 1494-1501.
Sang T, Crawford DJ, Stuessy TF, Silva O M.
1995. ITS sequences and the phylogeny of the genus Robinsonia (Asteraceae). – Syst. Bot. 20:
55-64.
Sanz M, Vilatersana R, Hidalgo O,
Garcia-Jacas N, Susanna A, Schneeweiss GM, Vallès J. 2008. Molecular phylogeny
and evolution of floral characters of Artemisia and allies
(Anthemideae, Asteraceae):
evidence from nrDNA ETS and ITS sequences. – Taxon 57: 66-78.
Sareedenchai V, Zidorn C. 2010. Flavonoids as
chemosystematic markers in the tribe Cichorieae of the Asteraceae. – Biochem. Syst. Ecol.
38: 935-987.
Sareedenchai V, Ganzera M, Ellmerer EP,
Lohwasser U, Zidorn C. 2009. Phenolic compounds from Tragopogon
porrifolius L. – Biochem. Syst. Ecol. 37: 234-236.
Saunders K. 2005. First record of
Nymphoides indica (Menyanthaceae) in Texas. – Sida
21: 2441-2443.
Savolainen V, Manen JF, Douzery E, Spichiger
R. 1994. Molecular phylogeny of families related to Celastrales
based on rbcL 5’ flanking sequences. – Mol. Phylogen. Evol. 3:
27-37.
Savolainen V, Spichiger R, Manen J-F. 1997.
Polyphyletism of Celastrales
deduced from a chloroplast noncoding DNA region. – Mol. Phylogen. Evol. 7:
145-157.
Schilling EE. 2011. Hybrid genera in
Liatrinae (Asteraceae:
Eupatorieae). – Mol. Phylogen. Evol. 59: 158-167.
Schilling EE, Jansen RK. 1989. Restriction
fragment analysis of chloroplast DNA and the systematics of Viguiera
and related genera (Asteraceae:
Heliantheae). – Amer. J. Bot. 76: 1769-1778.
Schilling EE, Panero JL. 1990. A new species
of Viguiera (Asteraceae:
Heliantheae) from Mexico. – Brittonia 42: 56-58.
Schilling EE, Panero JL. 1991. Evidence for a
close relationship between Iostephane and Viguiera (Asteraceae: Heliantheae). – Amer.
J. Bot. 78: 1054-1062.
Schilling EE, Panero JL. 1996. Phylogenetic
reticulation in subtribe Helianthinae. – Amer. J. Bot. 83: 939-948.
Schilling EE, Panero JL. 2002. A revised
classification of subtribe Helianthinae (Asteraceae-Heliantheae) I. Basal
lineages. – Bot. J. Linn. Soc. 140: 65-76.
Schilling EE, Schilling EM. 1986. Chromosome
numbers in Viguiera Series Dentatae (Compositae). – Syst.
Bot. 11: 51-55.
Schilling EE, Spooner DM. 1988. Floral
flavonoids and the systematics of Simsia (Asteraceae: Heliantheae). – Syst.
Bot. 13: 572-575.
Schilling EE, Panero JL, Eliasson UH. 1994.
Evidence from chloroplast DNA restriction site analysis on the relationships of
Scalesia (Asteraceae:
Heliantheae). – Amer. J. Bot. 81: 248-254.
Schilling EE, Linder CR, Noyes RD, Rieseberg
LH. 1998. Phylogenetic relationships in Helianthus (Asteraceae) based on nuclear
ribosomal DNA internal transcribed spacer region sequence data. – Syst. Bot.
23: 177-187.
Schilling EE, Panero JL, Cox PB. 1999.
Chloroplast DNA restriction site data support a narrowed interpretation of
Eupatorium (Asteraceae).
– Plant Syst. Evol. 219: 209-223.
Schilling EE, Panero JL, Crozier BS, Aranda
PD. 2013. Relationships of Asanthus (Asteraceae, Eupatorieae). – Syst.
Bot. 38: 253-258.
Schilling EE, Panero JL, Crozier BS, Scott
RW, Dávila P. 2015. Bricklebush (Brickellia) phylogeny reveals
dimensions of the great Asteraceae
radiation in Mexico. – Mol. Phylogen. Evol. 85: 161-170.
Schilling N. 1976. Distribution of
L-(+)-bornesitol in the Gentianaceae and Menyanthaceae. – Phytochemistry
15: 824-826.
Schmidt GJ, Schilling EE. 2000. Phylogeny and
biogeography of Eupatorium (Asteraceae: Eupatorieae) based on
nuclear ITS sequence data. – Amer. J. Bot. 87: 716-726.
Schmidt H. 1904. Systematisch-anatomische
Untersuchungen des Blattes der Campanuloideen. – Inaugural-Diss.,
Universität Erlangen, Germany.
Schmidt-Lebuhn AN. 2013. Reciprocal monophyly
of Craspedia and Pycnosorus (Asteraceae, Gnaphalieae) and the
problems of using ribosomal DNA at the lowest taxonomic levels. – Aust. Syst.
Bot. 26: 233-237.
Schmidt-Lebuhn AN, Milner KV. 2013. A
quantitative study of morphology in Australian Craspedia (Asteraceae, Gnaphalieae). – Aust.
Syst. Bot. 26: 238-254.
Schmidt-Lebuhn AN, Smith KJ. 2016. From the
desert it came: evolution of the Australian paper daisy genus
Leucochrysum (Asteraceae,
Gnaphalieae). – Aust. Syst. Bot. 29: 176-184.
Schmidt-Lebuhn AN, Bruhl JJ, Telford IRH,
Wilson PG. 2015. Phylogenetic relationships of Coronidium,
Xerochrysum and several neglected Australian species of
“Helichrysum” (Asteraceae: Gnaphalieae). – Taxon
64: 96-109.
Schnepf E. 1969. Über den Feinbau von
Öldrüsen I. Die Drüsenhaare von Arctium lappa. – Protoplasma 67:
185-194.
Schönland S. 1890. Notes on Cyphia
volubilis, Willd. – Trans. South Afr. Philos. Soc. 6: 44-51.
Schönland S. 1894a. Campanulaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien IV(5), W. Engelmann, Leipzig,
pp. 40-70, 394.
Schönland S. 1894b. Goodeniaceae. – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien IV(5), W. Engelmann, Leipzig,
pp. 70-79.
Schönland S. 1894c. Candolleaceae (Stylidiaceae). – In: Engler A,
Prantl K (eds), Die natürlichen Pflanzenfamilien IV(5), W. Engelmann, Leipzig,
pp. 79-84.
Schultheis LM. 2001. Systematics of
Downingia (Campanulaceae) based on molecular
sequence data: implications for floral and chromosome evolution. – Syst. Bot.
26: 603-621.
Schürhoff PN. 1926. Synergidenhaustorien der
Calenduleae und Arctotideae sowie die systematische Stellung der Compositae.
– Ber. Deutsch. Bot. Ges. 44: 665-673.
Schweizer D, Ehrendorfer F. 1983. Evolution
of C-band patterns in Asteraceae-Anthemideae. – Biol.
Zbl. 102: 637-655.
Scogin R. 1978. Floral UV-absorption patterns
and anthochlor pigments in the Asteraceae. – Southwest Natur. 23:
371-374.
Scott AJ. 1985. Microcharacters as generic
markers in the Eupatorieae. – Taxon 34: 26-30.
Scott AJ. 1987. Notes on Compositae: Inuleae
for the ‘Flore des Mascareignes’. – Kew Bull. 42: 854.
Scott AJ. 1991. Notes on Compositae-Astereae
for the ‘Flore des Mascareignes’. – Kew Bull. 46: 339-353.
Scott AJ. 1997. 85. Brexiacées. – In:
Bosser J, Cadet T, Guého J, Marais W (eds), Flore des Mascareignes, Imprimerie
de Montligeon, La Chapell Montligeon.
Scott L, Cadman A, McMillan I. 2006. Early
history of Cainozoic Asteraceae
along the southern African west coast. – Rev. Palaeobot. Palynol. 142:
47-52.
Seaman FC. 1982. Sesquiterpene lactones as
taxonomic characters in the Asteraceae. – Bot. Rev., N. S., 48:
121-194.
Seaman FC, Funk VA. 1983. Cladistic analysis
of complex natural products: developing transformation series from
sesquiterpene lactone data. – Taxon 32: 1-27.
Seaman FC, Mabry TJ. 1979. Sesquiterpene
lactones and species relationships among the shrubby Ambrosia taxa.
– Biochem. Syst. Ecol. 7: 105-114.
Seaman FC, Fisher NH, Stuessy TF. 1980.
Systematic implications of sesquiterpene lactones in the subtribe
Melampodiinae. – Biochem. Syst. Ecol. 8: 263-271.
Seaman FC, Bohlmann H, Zdero C, Mabry TJ.
1990. Diterpenes of flowering plants: Compositae (Asteraceae). – Springer, New
York.
Seeligmann P. 1996. Flavonoids of the
Compositae as evolutionary parameters in the tribes which synthesize them: a
critical approach. – In: Hind DJN, Beentje HJ (eds), Compositae: systematics.
Proceedings of the International Compositae Conference, Kew, 1994, Royal
Botanic Garden, Kew, pp. 159-167.
Seguineau M-F, Langlois N. 1980.
Phellibilidine, nouvel alcaloïde de Phelline billardieri. –
Phytochemistry 19: 1279-1281.
Sehgal D, Raina SN, Devarumath RM, Sasanuma
T, Sasakuma T. 2009. Nuclear DNA assay ion solving issues related to ancestry
of the domesticated diploid safflower (Carthamus tinctorius L.) and
the polyploidy (Carthamus) taxa, and phylogenetic and genomic
relationships in the genus Carthamus L. – Mol. Phylogen. Evol. 53:
631-644.
Seidenschnur C, Beaman J. 1966.
Cuchumatanea – a new genus of the Compositae (Heliantheae). –
Rhodora 68: 139-146.
Seigler DS, Wilken DH, Jakupcak JJ. 1974.
Chemical data related to the tribal affinities of Hulsea and
Arnica. – Biochem. Syst. Ecol. 2: 21-24.
Sell PD. 1975. Taxonomic and nomenclatural
notes on the Compositae subfamily Cichorioideae. – Bot. J. Linn. Soc.
71:236-274.
Semple JC. 1977a. Chromosome numbers and
karyotypes in Borrichia (Compositae). – Syst. Bot. 2: 287-291.
Semple JC. 1977b. Cytotaxonomy of
Chrysopsis and Heterotheca (Compositae-Astereae): a new
interpretation of phylogeny. – Can. J. Bot. 55: 2503-2513.
Semple JC. 1981. A revision of the
goldenaster genus Chrysopsis (Nutt.) Ell. nom. cons.
(Compositae-Astereae). – Rhodora 83: 323-384.
Semple JC. 1982. Observations on morphology
and cytology of Aster hemisphaericus, A. paludosus, and
A. chapmanii (Asteraceae)
with comments on chromosomal base number and phylogeny of Aster subg.
Aster sect. Heleastrum. – Syst. Bot. 7: 60-70.
Semple JC. 2006. Quadruple, triple, double,
and simple pappi in the goldenasters, subtribe Chrysopsidinae (Asteraceae: Astereae). – Sida 22:
503-531.
Semple JC, Bowers FD. 1985. A revision of the
goldenaster genus Pityopsis Nutt. (Compositae-Astereae). – Univ.
Waterloo Biol. Ser. 29: 1-34.
Semple JC, Brouillet L. 1980a. A synopsis of
North American asters: the subgenera, sections, and subsections of
Aster and Lasallea. – Amer. J. Bot. 67: 1010-1026.
Semple JC, Brouillet L. 1980b. Chromosome
numbers and satellite chromosome morphology in Aster and
Lasallea. – Amer. J. Bot. 67: 1027-1039.
Semple JC, Chinnappa CC. 1980. Karyotype
evolution and chromosome numbers in Chrysopsis (Nutt.) Ell. sensu
Semple (Compositae-Astereae). – Can. J. Bot. 58: 164-171.
Semple JC, Chinnappa CC. 1984. Observations
on the cytology, morphology, and ecology of Bradburia hirtella
(Compositae-Astereae). – Syst. Bot. 9: 95-101.
Semple JC, Watanabe K. 2009. A review of
chromosome numbers in Asteraceae
with hypotheses on chromosomal base number evlution. – In: Funk VA, Susanna
A, Stuessy TF, Bayer RJ (eds), Systematics, evolution, and biogeography of
Compositae, International Association for Plant Taxonomists, Wien, pp.
61-72.
Semple JC, Blok VC, Heiman P. 1980.
Morphological, anatomical, habit, and habitat differences among the goldenaster
genera Chrysopsis, Heterotheca, and Pityopsis
(Compositae-Astereae). – Can. J. Bot. 58: 147-163.
Semple JC, Leeder C, Leuty C, Gray L. 1988.
Heterotheca sect. Ammodia (Compositae: Astereae): a
multivariate study of H. oregona and specimens of Brewer’s (golden)
aster. – Syst. Bot. 13: 547-558.
Senianinova-Korchagina MV. 1952. On the
inferior ovary in Compositae. – Bull. Moscow Soc. Nat., Biol. Section, n. s.,
57(4): 63-75.
Sennikov AN. 1997. Vidy roda
Prenanthes i gruppy rodov iz rodstva Cicerbita (Asteraceae) na Kavkaze. – Bot.
Žurn. 82: 106-114.
Sennikov AN. 2000. O rodakh iz rodstva
Prenanthes L. (Asteraceae). – Novosti Sist. Vysš.
Rast. 32: 178-181.
Sennikov AN. 2010. A revision of
Cousinia sections Alpinae (syn. Carduncellus),
Subappendiculatae and Tianschanicae (Asteraceae) in the Kirghizian
Tian-Shan and the neighbouring territories. – Phytotaxa 5: 1-30.
Sennikov AN, Illarinova ID. 2001. The
morphological and anatomical structure of the achenes of species of the genus
Hieracium (Asteraceae)
and related genera. – Bot. Žurn. 86: 37-59. [In Russian]
Shannon RK, Wagner WL. 1997.
Oparanthus revisited. – In: Lorence D (ed), Botanical results of the
1988 Fatu Hiva Expedition to the Marquesas Islands. – Allertonia 7:
273-295.
Sheidai M, Nasirzadeh A, Kheradnam M. 2000.
Karyotypic study of Echinops (Asteraceae) in Fars Province, Iran.
– Bot. J. Linn. Soc. 134: 453-463.
Shen LD, Tang XY, Yue SJ, Xie CK. 1975. The
genus Codonopsis Wall. in Szechuan. – Acta Phytotaxon. Sin. 13:
55-68.
Sherff EE. 1923. New or otherwise noteworthy
Compositae. – Bot. Gaz. 76: 78-79.
Sherff EE. 1926. Revision of the genus
Isostigma Less. – Bot. Gaz. 81: 241-257.
Sherff EE. 1931. New or otherwise noteworthy
Compositae VI. – Bot. Gaz. 91: 312-314.
Sherff EE. 1935a. New or otherwise noteworthy
Compositae X. – Amer. J. Bot. 22: 705-710.
Sherff EE. 1935b. Revision of
Tetramolopium, Lipochaeta, Dubautia, and
Railliardia. – Bull. B. P. Bishop Mus. 135: 1-136.
Sherff EE. 1940. A new genus of Compositae
from northwestern Alabama. – Field Mus. Nat. Hist. Bot. Ser. 22: 399-403.
Sherff EE. 1947. Additions to the genera
Scalesia Arn. and Hidalgoa Llave and Lex. – Field Mus. Nat.
Hist. Bot. Ser. 23: 333-334.
Sherman M. 1946. Karyotype evolution: a
cytogenetic study of seven species and six interspecific hybrids of
Crepis. – Univ. Calif. Publ. Bot. 18: 369-408.
Shetler SG. 1963. A checklist and key to the
species of Campanula native or commonly naturalized in North America.
– Rhodora 65: 319-337.
Shetler SG. 1979. Pollen-collecting hairs of
Campanula (Campanulaceae) I. Historical
review. – Taxon 28: 205-215.
Shetler SG, Morin NR. 1986. Seed morphology
in North American Campanulaceae. – Ann. Missouri
Bot. Gard. 73: 653-688.
Shibayama Y, Kadono Y. 2003. Floral morph
composition and pollen limitation in the seed set of Nymphoides indica
populations. – Ecol. Res. 18: 725-737.
Shih C. 1987. On the circumscription of the
genus Prenanthes L. and Notoseris Shih: a new genus of
Compositae from China. – Acta Phytotaxon. Sin. 25: 189-203.
Shih C. 1988. Revision of Lactuca L.
and two new genera of tribe Lactuceae (Compositae) on the mainland of Asia. –
Acta Phytotaxon. Sin. 26: 418-428.
Shih C. 1991. On circumscription of the genus
Cicerbita Wall., and two new genera of Compositae from Sino-Himalayan
region. – Acta Phytotaxon. Sin. 29: 394-417.
Shih C, Chen Y-L. 1996. Faberiopsis
Shih et Y.L. Chen, genus novum familiae Compositarum sinensium. – Acta
Phytotaxon. Sin. 34: 438-439.
Shinners LH. 1946a. Revision of the genus
Leucelene Greene. – Wrightia 1: 82-89.
Shinners LH. 1946b. Revision of the genus
Aphanostephus DC.; the genus Dichaetophora A. Gray and its
relationships. – Wrightia 1: 90-121.
Shinners LH. 1949. Revision of the genus
Egletes Cassini North of South America. – Lloydia 12: 239-250.
Shore BF. 1969. Dioecism in New Zealand Escalloniaceae.
– New Zealand J. Bot. 7: 113-124.
Short PS. 1983. A revision of
Angianthus Wendl., sensu lato (Compositae: Inuleae: Gaphalieae) 1. –
Muelleria 5: 143-183.
Short PS. 1989. New genera and species of
Australian Inuleae (Asteraceae).
– Muelleria 7: 103-116.
Short PS. 1990. New taxa and new combinations
in Australian Gnaphaliinae (Inuleae: Asteraceae). – Muelleria 7:
239-252.
Short PS. 1995. A revision of
Millotia (Asteraceae-Gnaphalieae). – Aust.
Syst. Bot. 8: 1-47.
Short PS. 2000a. Notes on
Myriocephalus Benth. s. lat. (Asteraceae: Gnaphalieae). – Aust.
Syst. Bot. 13: 729-738.
Short PS. 2000b. Two new species of
Asteridea Lindl. (Asteraceae: Gnaphalieae). – Aust.
Syst. Bot. 13: 739-744.
Short PS, Anderberg AA. 1995. A cladistic
analysis of Millotia Cass. (Asteraceae-Gnaphalieae). – Aust.
Syst. Bot. 8: 49-55.
Short PS, Watanabe K. 1993. Two new species
of Brachyscome (Asteraceae: Astereae) from eastern
Australia. – Aust. Syst. Bot. 6: 335-342.
Short PS, Wilson PG. 1990. Haegiela,
a new genus of Australian Asteraceae (Inuleae:Gnaphaliinae),
with notes on the genus Epaltes Cass. – Muelleria 7: 259-265.
Short PS, Wilson KE, Nailon J. 1989. Notes on
the fruit anatomy of Australian members of the Inuleae (Compositae). –
Muelleria 7: 57-79.
Shrestha KK. 1992. Taxonomic revision of the
genus Cyananthus Wall. ex Benth. (Campanulaceae). – Ph.D. diss.,
University of S:t Petersburg, Russia.
Shrestha KK. 1997. Taxonomic revision of the
Sino-Himalayan genus Cyananthus (Campanulaceae). – Acta
Phytotaxon. Sin. 35: 396-433.
Shrestha KK, Kravtsova TI. 1992. Seed coat
anatomy and ultrastructure in the genus Cyananthus in relation to its
systematics. – Bot. Žurn. 77: 18-29. [In Russian with English summary]
Shrestha KK, Tarasevich VF. 1992. Comparative
pollen morphology of the genus Cyananthus in relation to its
systematics and its position within the family Campanulaceae. – Bot. Žurn. 77:
1-13. [In Russian with English summary]
Shulkina TV. 1978. Life forms in Campanulaceae, their geographical
distribution and connection with taxonomy. – Bot. Žurn. 63: 153-169. [In
Russian]
Shulkina TV. 1979. De positione systematica
Campanula lactiflora Bieb. – Novosti Sist. Vyssh. Rast. 16: 175-179.
[In Russian]
Shulkina TV. 1980. The significance of
life-form characters for systematics, with special reference to the family Campanulaceae. – Plant Syst.
Evol. 136: 233-246.
Shulkina TV, Zykov SE. 1980. The anatomical
structure of the stem in the Campanulaceae s. str. in relation
to the evolution of life forms. – Bot. Žurn. 65: 627-638.
Shulkina TV, Gaskin JF, Eddie WMM. 2003.
Morphological studies toward an improved classification of Campanulaceae s. str. – Ann.
Missouri Bot. Gard. 90: 576-591.
Shultz LM. 2009. Monograph of
Artemisia Subgenus Tridentatae (Asteraceae-Anthemideae). – Syst.
Bot. Monogr. 89: 1-131.
Shumovich W, Montgomery FH. 1955. The
perennial sow thistles in northeastern North America. – Can. J. Agric. Sci.
35: 601-605.
Sieren DJ. 1981. The taxonomy of the genus
Euthamia. – Rhodora 83: 551-579.
Silva TDG, Marzinek J, Hattori EKO, Nakajima
JN, De-Paula OC. 2018. Comparative cypsela morphology in Disynaphiinae and
implications for their systematics and evolution (Eupatorieae: Asteraceae). – Bot. J. Linn. Soc.
186: 89-107.
Simões-Pires CA, Debenedetti S, Spegazzini
E, Mentz LA, Matzenbacher NI, Limberger RP, Henriques AT. 2005. Investigation
of the essential oil from eight species of Baccharis belonging to
sect. Caulopterae (Asteraceae, Astereae): a taxonomic
approach. – Plant Syst. Evol. 253: 23-32.
Simon VC. 1978. Bellium corsicum n.
sp. sowie Notizen über die Gattung Bellium (Asteraceae). – Bauhinia 6:
279-284.
Simpson BB. 1975. XII. Mutisieae. – In:
Woodson RE et al. (eds), Flora of Panama, Ann. Missouri Bot. Gard. 62:
1276-1291.
Simpson BB, Arroyo MTK, Sipe S, Dias de
Morães M, McDill J. 2009. Phylogeny and evolution of Perezia (Asteraceae: Mutisieae: Nassauviinae).
– J. Syst. Evol. 47: 431-443.
Simurda MC, Marshall DC, Knox JS. 2005.
Phylogeography of the narrow endemic, Helenium virginicum (Asteraceae), based upon ITS sequence
comparisons. – Syst. Bot. 30: 887-898.
Singh D, Kaul V, Dathan ASR. 1974.
Cytological studies in the genus Taraxacum Weber. – Proc. Indian
Acad. Sci., Sect. B, 80: 82-91.
Sivarajan VV, Joseph KT. 1993. The genus
Nymphoides Séguier (Menyanthaceae) in India. –
Aquatic Bot. 45: 145-170.
Sivarajan VV, Chaw S-M, Joseph KT. 1989. Seed
coat micromorphology of Indian species of Nymphoides (Menyanthaceae). – Bot. Bull.
Acad. Sin. 30: 275-283.
Skottsberg C. 1915. Notes on the relations
between the floras of Subantarctic America and New Zealand. – The Plant World
18: 129-142.
Skvarla JJ, Larson DA. 1965. An electron
microscope study of pollen morphology in the Compositae with special reference
to the Ambrosiinae. – Grana Palynol. 6: 210-269.
Skvarla JJ, Turner BL. 1966a. Systematic
implications from electron microscopic studies of Compositae pollen – a
review. – Ann. Missouri Bot. Gard. 53: 220-256.
Skvarla JJ, Turner BL. 1966b. Pollen wall
ultrastructure and its bearing on the systematic position of
Blennosperma and Crocidium (Compositae). – Amer. J. Bot.
53: 555-563.
Skvarla JJ, Turner BL. 1969. Fine structure
of Petrobinae (Compositae-Heliantheae) pollen walls. – Amer. J. Bot. 56:
418-419.
Skvarla JJ, Turner Bl. 1971. Fine structure
of the pollen of Anthemis nobilis L. (Anthemideae-Compositae). –
Proc. Oklahoma Acad. Sci. 51: 61-62.
Skvarla JJ, Turner BL, Patel VC, Tomb AS.
1976. Review of Compositae pollen morphology and families suggesting
morphological relationships. – In: Heywood VH, Harborne J, Turner BL (eds),
Systematics and chemistry of Compositae I, Academic Press, London, pp.
141-248.
Skvarla JJ, Turner BL, Patel VC, Tomb AS.
1977. Pollen morphology in the Compositae and in morphologically related
families [Appendix: Thanikaimoni G, Principal works on the pollen morphology of
Compositae]. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae, Academic Press, London, pp. 141-265.
Skvarla JJ, DeVore ML, Chissoe WF. 2005.
Lophate sculpturing of Vernonieae (Compositae) pollen. – Rev. Palaeobot.
Palyn. 133: 51-68.
Slooten DF van. 1937. The Stylidiaceae of the Netherlands
Indies. – Bull. Jard. Bot. Buitenzorg, Ser. III, 14: 169-174.
Slooten DF van. 1954. Stylidiaceae. – In: Steenis CGGJ
van (ed), Flora Malesiana, I, 4(5), Noordhoff-Kolff N.V., Batavia, pp.
529-532.
Slovák M, Singliarová B, Mráz P. 2007.
Chromosome numbers and mode of reproduction in Picris hieracioides
s.l. (Asteraceae), with notes on
some other Picris taxa. – Nord. J. Bot. 25: 238-244.
Small J. 1917. The origin and development of
the Compositae. – New Phytol. 16: 157-177, 198-221, 253-276.
Small J. 1918. The origin and development of
the Compositae. – New Phytol. 17: 13-40, 69-94, 114-142, 200-230.
Small J. 1919. The origin and development of
the Compositae. – New Phytol. 18: 1-35, 65-89, 129-176, 201-234.
Small J. 1919. The origin and development of
the Compositae. – William Wesley & Son, London.
Smalla M. 2000. Studies in the Compositae of
the Arabian Peninsula and Socotra 6. The Hypochaeridinae (Lactuceae) in the
Arabian Peninsula. – Willdenowia 30: 315-337.
Smissen RD, Breitwieser I, Ward JM. 2004.
Phylogenetic implications of trans-specific chloroplast DNA sequence
polymorphism in New Zealand Gnaphalieae (Asteraceae). – Plant Syst. Evol.
249: 37-53.
Smissen RD, Galbany-Casals M, Breitwieser I.
2011. Ancient allopolyploidy in the everlasting daisies (Asteraceae: Gnaphalieae): complex
relationships among extant clades. – Taxon 60: 649-662.
Smith BN, Turner BL. 1975. Distribution of
Kranz syndrome among Asteraceae.
– Amer. J. Bot. 62: 541-545.
Smith Jr CE. 1969. Pollen characteristics of
African species of Vernonia. – J. Arnold Arbor. 50: 469-477.
Smith Jr CE. 1971. Observations on
stengelioid species of Vernonia. – Agriculture Handbook 396.
Agriculture Res. Serv., U. S. Dept. Agriculture, Washington, D.C.
Smith EB. 1965. Taxonomy of
Haplopappus, section Isopappus (Compositae). – Rhodora 67:
217-238.
Smith EB. 1966. Cytogenetics and phylogeny of
Haplopappus sect. Isopappus (Compositae). – Can. J. Genet.
Cytol. 8: 14-36.
Smith EB. 1975. The chromosome numbers of
North American Coreopsis with phyletic interpretations. – Bot. Gaz.
136: 78-86.
Smith EB. 1981. New combinations in
Croptilon (Compositae-Astereae). – Sida 9: 59-63.
Smith EB. 1989. A biosystematic study and
revision of the genus Coreocarpus (Compositae). – Syst. Bot. 14:
448-472.
Smith LS. 1958. Corokia A. Cunn. An
addition to the Australian genera of Saxifragaceae.
– Proc. Roy. Soc. Queensland 69: 53-55.
Smith PJ. 1992. A revision of the genus
Wahlenbergia (Campanulaceae) in Australia. –
Telopea 5: 91-175.
Smith SA, Beaulieu JM, Donoghue MJ. 2010. An
uncorrelated relaxed-clock analysis suggests an earlier origin for flowering
plants. – Proc. Natl. Acad. Sci. U.S.A. 107: 5897-5902.
Smith SA, Beaulieu JM, Stamatakis A, Donoghue
MJ. 2011. Understanding angiosperm diversification using small and large
phylogenetic trees. – Amer. J. Bot. 98: 404-414.
Smith-White SC, Carter CR, Stace HM. 1970.
The cytology of Brachycome I. The subgenus Eubrachycome: a
general survey. – Aust. J. Bot. 18: 99-125.
Smoljaninova LA. 1955. De genere
Micropus L. notulae systematicae. – Bot. Mater. Gerb. Bot. Inst.
Komarova Akad. Nauk SSSR 17: 447-454.
Smoljaninova LA. 1960. Note de genre
Symphyllocarpus Maxim. – Bot. Mater. Gerb. Bot. Inst. Komarova Akad.
Nauk S.S.S.R. 20: 282-288.
Snogerup B. 1980. The genus
Reichardia (Asteraceae)
in the Aegean area. – Bot. Not. 133: 515-520.
Soejima A, Yahara T, Watanabe K. 2001.
Thirteen new species and two new combinations of Stevia (Asteraceae: Eupatorieae) from Mexico.
– Brittonia 53: 377-395.
Soejima A, Wen J, Zapata M, Dillon MO. 2008.
Phylogeny and putative hybridization in the subtribe Paranepheliinae (Liabeae,
Asteraceae), implications for
classification, biogeography, and Andean orogeny. – J. Syst. Evol. 46:
375-390.
Solbrig OT. 1960a. Cytotaxonomic and
evolutionary studies in the North American species of Gutierrezia. –
Contr. Gray Herb. 188: 1-63.
Solbrig OT. 1960b. The South American
sections of Erigeron and their relation to Celmisia. –
Contr. Gray Herb. 188: 65-85.
Solbrig OT. 1960c. The status of the genera
Amphipappus, Amphiachyris, Greenella,
Gutierrezia, Gymnosperma and Xanthocephalum
(Compositae). – Rhodora 62: 43-54.
Solbrig OT. 1962. The South American species
of Erigeron. – Contr. Gray Herb. 191: 3-79.
Solbrig OT. 1963. Subfamilial nomenclature of
Compositae. – Taxon 12: 229-235.
Solbrig OT. 1966. The South American species
of Gutierrezia. – Contr. Gray Herb. 197: 3-42.
Solbrig OT. 1970. The phylogeny of
Gutierrezia: an eclectic approach. – Brittonia 22: 217-229.
Solbrig OT. 1977. Chromosomal cytology and
evolution in the family Compositae. – In: Heywood VH, Harborne JB, Turner BL
(eds), The biology and chemistry of the Compositae, Academic Press, London, pp.
267-281.
Solbrig OT, Anderson LC, Kyhos DW, Raven PH,
Rüdenberg L. 1964. Chromosome numbers in Compositae V. Astereae II. – Amer.
J. Bot. 51: 513-519.
Soltis DE, Soltis PS. 1989. Allopolyploid
speciation in Tragopogon: insights from chloroplast DNA. – Amer. J.
Bot. 76: 1119-1124.
Soltis DE, Soltis PS. 1997. Phylogenetic
relationships in Saxifragaceae
sensu lato: a comparison of topologies based on 18S rDNA and rbcL
sequences. – Amer. J. Bot. 84: 504-522.
Soltis DE, Soltis PS, Clegg MT, Durbin M.
1990. rbcL sequence divergence and phylogenetic relationships in Saxifragaceae
sensu lato. – Proc. Natl. Acad. Sci. U.S.A. 87: 4640-4644.
Soltis, PS, Soltis DE, Doyle JJ (eds). 1992.
Molecular systematics of plants. – Chapman and Hall, New York.
Soltis PS, Plunkett GM, Novak SJ, Soltis DE.
1995. Genetic variation in Tragopogon species: additional origins of
the allotetraploids T. mirus and T. miscellus (Compositae).
– Amer. J. Bot. 82: 1329-1341.
Sonboli A, Stroka K, Kazempour Osaloo S,
Oberprieler C. 2012. Molecular phylogeny and taxonomy of Tanacetum L.
(Compositae, Anthemideae) inferred from nrDNA ITS and cpDNA
trnH/psbA sequence variation. – Plant Syst. Evol. 298:
431-444.
Soreng RJ, Cope EA. 1991. On the taxonomy of
cultivated species of the Chrysanthemum genus-complex (Anthemideae;
Compositae). – Baileya 23: 145-165.
Soreng RJ, Spellenberg RW. 1984. An unusual
new Chaetopappa (Asteraceae-Astereae) from New Mexico.
– Syst. Bot. 9: 1-5.
Sørensen NA. 1977. Polyacetylenes and
conservatism of chemical characters in the Compositae. – In: Heywood VH,
Harborne JB, Turner BL (eds), The biology and chemistry of the Compositae,
Academic Press, London, pp. 385-409.
Sosnovsky D. 1931. On two sections of the
genus Centaurea L. s. str. – Věstn. Tiflissk. Bot. Sada, ser. II,
5: 21-34. [In Russian]
Sosnovsky D. 1948. Specierum caucasicarum
generis Psephelli (Cass.) D. Sosn. emend. revisio. – Zametki Sist.
Geogr. Rast. 14: 5-22. [In Russian]
Sosnovsky D. 1953. Prodromus generis
Aetheopappi Cass. – Zametki Sist. Geogr. Rast. 17: 4-16.
Sosnovsky D, Takhtajan AL. 1945. The revision
of the Caucasian representatives of Centaureinae II. On the new genus
Grossheimia Sosn. & Takht. – Proc. Acad. Sci. Armen. SSR 3: 22.
[In Russian]
Sosnowec AA. 1960. K citologii roda
Scorzonera L. – Bot. Žurn. 45: 1813-1815.
Soto-Trejo F, Schilling EE, Solórzano S,
Oyama K, Lira R, Dávila P. 2015. Phylogenetic relationships in the genus
Florestina (Asteraceae,
Bahieae). – Plant Syst. Evol. 301: 2147-2160.
Souèges R. 1938. Embryogénie des
Campanulacées. Développement de l’embryon chez le Campanula patula
L. – Compt. Rend. Acad. Sci. Paris 202: 2009-2011.
Southworth D. 1983. Exine development in
Gerbera jamesonii (Asteraceae: Mutisieae). – Amer. J.
Bot. 70: 1038-1047.
Spongberg SA. 1971. A systematic and
evolutionary study of North American arctic and alpine monocephalous species of
Erigeron (Compositae). – Ph.D. diss., University of North Carolina,
Chapel Hill, North Carolina.
Spooner DM. 1990. Systematics of
Simsia (Compositae: Heliantheae). – Syst. Bot. Monogr. 30: 1-90.
Spooner DM, De Jong DCD, Sun B-Y, Stuessy TF,
Gengler KM, Nesom GL, Berry PE. 1995. Chromosome counts of Compositae from
Ecuador and Venezuela. – Ann. Missouri Bot. Gard. 82. 596-602.
Stace HM. 1978. Cytoevolution in the genus
Calotis R. Br. (Compositae: Astereae). – Aust. J. Bot. 26:
287-307.
Stace HM. 1981. Biosystematics of the
Brachyscome aculeata species complex (Compositae: Astereae). – Aust.
J. Bot. 29: 425-440.
Stace HM, James SH. 1996. Another perspective
on cytoevolution in Lobelioideae (Campanulaceae). – Amer. J. Bot.
83: 1356-1364.
Stångberg F, Anderberg AA. 2014. Morphology
and taxonomic reclassification of Gorteria (Asteraceae). – Willdenowia 44:
97-120.
Stångberg F, Ellis AG, Anderberg AA. 2013.
Evolutionary relationships in Gorteria: a re-evaluation. – Taxon 62:
537-549.
Stebbins GL. 1937a. Critical notes on
Lactuca and related genera. – J. Bot. (London) 75: 12-18.
Stebbins GL. 1937b. The scandent species of
Prenanthes and Lactuca. – Bull. Jard. Bot. État 14:
333-352.
Stebbins GL. 1939. Notes on some Indian
species of Lactuca. – Indian For. Bot. 1: 237-245.
Stebbins GL. 1940. Studies in Cichorieae:
Dubyaea and Soroseris. Endemics of the Sino-Himalayan region.
– Mem. Torrey Bot. Club 19: 1-76.
Stebbins GL. 1953a. A new classification of
the tribe Cichorieae, family Compositae 1. – Madroño 12: 33-64.
Stebbins GL. 1953b. A new classification of
the tribe Cichorieae, family Compositae 2. – Madroño 12: 65-81.
Stebbins GL. 1953c. Subfamilial nomenclature
of Compositae. – Taxon 12: 229-235.
Stebbins GL. 1977. Developmental and
comparative anatomy of the Compositae. – In: Heywood VH, Harborne JB, Turner
BL (eds), The biology and chemistry of the Compositae, Academic Press, London,
pp. 91-109.
Stebbins GL, Jenkins JA, Walters MS. 1953.
Chromosomes and phylogeny in the Compositae, tribe Cichorieae. – Univ. Calif.
Publ. Bot. 26: 401-429.
Steenis CGGJ van. 1978. The genus
Periomphale in New Guinea (Caprifoliaceae). –
Blumea 24: 480-481.
Steenis CGGJ van. 1982. 157. Preliminary note
on the taxonomic disposition of Platyspermation Guillaumin (Myrtaceae) from New
Caledonia. – In: Steenis CGGJ van, Veldkamp JF (eds), Miscellaneous botanical
notes XXVI, Reinwardtia 10: 21-26.
Steenis CGGJ van. 1984. A synopsis of Alseuosmiaceae in New Zealand,
New Caledonia, Australia, and New Guinea. – Blumea 29: 387-394.
Steenis CGGJ van. 1986. Alseuosmiaceae. – In: Steenis
CGGJ van, Wilde WJJO de (eds), Flora malesiana I, 10(2), Martinus Nijhoff, The
Hague, Boston, London, pp. 335-336.
Stefanović S, Lakušić D, Kuzmina M,
Medeović S, Tan K, Stevanović V. 2008. Molecular phylogeny of
Edraianthus (grassy bells; Campanulaceae) based on non-coding
plastid DNA sequences. – Taxon 57: 452-475.
Steffen S, Dillenberger MS, Kadereit JW.
2016. Of dwarfs and giants: phylogeny of the Petasites-clade (Asteraceae-Senecioneae) and evolution
of miniaturization in arctic-alpine environments. – Plant Syst. Evol. 302:
545-559.
Stein BA. 1987a. Siphocampylus
oscitans (Campanulaceae:
Lobelioideae), a new name for Burmeistera weberbaueri from Peru. –
Ann. Missouri Bot. Gard. 74: 491-493.
Stein BA. 1987b. Synopsis of the genus
Burmeistera (Campanulaceae, Lobelioideae) in
Peru. – Ann. Missouri Bot. Gard. 74: 494-496.
Stein BA. 1987c. Systematics and evolution of
Centropogon subg. Centropogon (Campanulaceae: Lobelioideae). –
Ph.D. diss., Washington University, St. Louis, Missouri.
Steyermark JA. 1934. Studies in
Grindelia I. New species, varieties, and combinations of
Grindelia. – Ann. Missouri Bot. Gard. 21: 227-230.
Stille JS, Stuessy TF, Dickoré WB, Jaeger M,
Gemeinholzer B, Wisseman V. 2016. Comparative pappus micromorphology of
edelweiss (Leontopodium, Gnaphalieae, Asteraceae) with implications for
taxonomy, ecology and evolution. – Bot. J. Linn. Soc. 182: 612-636.
Stirton CH. 1983. Nocturnal petal movements
in the Asteraceae. – Bothalia
14: 1003-1006.
Stix H. 1960. Pollenmorphologische
Untersuchungen an Compositen. – Grana Palynol. 2: 41-114.
St. John H. 1950. Hawaiian plant studies 18.
The subgenera of Dubautia (Compositae). – Pacific Sci. 4:
339-345.
St. John H. 1971. The status of the genus
Wilkesia (Compositae) and discovery of a second Hawaiian species. –
Occas. Papers B. P. Bishop Mus. 24(8): 128-137.
St. John H. 1983. Hawaiian plant studies 114.
Hawaiian novelties in Clermontia (Lobeliaceae). – Nord. J. Bot. 3:
543-545.
Stolt KAH. 1921. Zur Embryologie der
Gentianaceen und Menyanthaceen. – Kungl. Sv. Vetensk.-Akad. Handl. 61(14):
3-56.
Straka H, Friedrich B. 1988. Palynologica
Madagassica et Mascarenica. Familien 65 bis 97. – Trop. Subtrop. Pflanzenwelt
61: 1-117.
Strijk JS, Noyes RD, Strasberg D, Cruaud C,
Gavory F, Chase MW, Abbott RJ, Thébaud C. 2012. In and out of Madagascar:
dispersal to peripheral islands, insular speciation and diversification of
Indian Ocean dasy trees (Psiadia, Asteraceae). – PloS One 7,
e42932.
Strother JL. 1966. Chromosome numbers in
Hymenoxys (Compositae). – Southwest. Natur. 11: 223-227.
Strother JL. 1977. Tageteae – systematic
review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae 2, Academic Press, London, pp. 769-783.
Strother JL. 1983. More chromosome numbers in
Compositae. – Amer. J. Bot. 70: 1217-1224.
Strother JL. 1986. Renovation of
Dyssodia (Compositae: Tageteae). – Sida 11: 371-378.
Strother JL. 1987. Damnxanthodium
(Compositae: Heliantheae), a new genus from Mexico. – Syst. Bot. 12:
41-43.
Strother JL. 1989a. Oblivia, a new
genus for Zexmenia mikanioides (Compositae: Heliantheae). – Syst.
Bot. 14: 541-543.
Strother JL. 1989b. Expansion of
Lundellianthus (Compositae: Heliantheae). – Syst. Bot. 14:
544-548.
Strother JL. 1991. Taxonomy of
Complaya, Elaphandra, Iogeton, Jefea,
Wamalchitamia, Wedelia, Zexmenia and
Zyzyxia (Compositae-Heliantheae-Ecliptinae). – Syst. Bot. Mongr. 33:
1-111.
Strother JL. 2001. Hedosyne
(Compositae, Ambrosiinae), a new genus for Iva ambrosiifolia –
Madroño 47: 204.
Strother JL. 2001. Nomenclatural status of
Carphobolus (Compositae: Vernonieae). – Taxon 50: 191-192.
Strother JL, Panero JL. 1994. Chromosome
studies: Latin American Compositae. – Amer. J. Bot. 81: 770-775.
Strother JL, Pilz G. 1975. Taxonomy of
Psathyrotes (Compositae: Senecioneae). – Madroño 23: 24-40.
Stucky J, Jackson RC. 1975. DNA content of
seven species of Astereae and its significance to theories of chromosome
evolution in the tribe. – Amer.J. Bot. 62: 509-518.
Stuessy TF. 1970a. The genus
Acanthospermum (Compositae-Heliantheae-Melampodinae): taxonomic
changes and generic affinities. – Rhodora 72: 106-109.
Stuessy TF. 1970b. Chromosome studies in
Melampodium (Compositae, Heliantheae). – Madroño 20: 365-372.
Stuessy TF. 1970c. Six new species of
Melampodium (Compositae: Heliantheae) from Mexico and Central America.
– Brittonia 22: 112-124.
Stuessy TF. 1973. A systematic review of the
subtribe Melampodiinae (Compositae, Heliantheae). – Contr. Gray Herb. Harvard
Univ. 203: 65-85.
Stuessy TF. 1976. A systematic review of the
subtribe Lagasceinae (Compositae, Heliantheae). – Amer. J. Bot. 63:
1289-1294.
Stuessy TF. 1977. Heliantheae – systematic
review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae II, Academic Press, London, pp. 621-671.
Stuessy TF. 2010. The rise of sunflowers. –
Science 329: 1605-1606.
Stuessy TF, Garver D. 1996. The defensive
role of pappus in heads of Compositae. – In: Caligari PDS, Hind DJN (eds),
Compositae: biology and utilization. Proceeding of the International Compositae
Conference, Kew, 1994, vol. 2, Royal Botanic Gardens, Kew, pp. 81-91.
Stuessy TF, Liu H-Y. 1983. Anatomy of the
pericarp of Clibadium, Desmanthodium, and
Ichthyothere (Compositae: Heliantheae) and systematic implications.
– Rhodora 85: 213-227.
Stuessy TF, Spooner DM. 1988. The adaptive
and phylogenetic significance of receptacular bracts in the Compositae. –
Taxon 37: 114-126.
Stuessy TF, Urtubey E. 2006. Phylogenetic
implications of corolla morphology in subfamily Barnadesioideae (Asteraceae). – Flora 201:
340-352.
Stuessy TF, Irving RS, Ellison WL. 1973.
Hybridization and evolution in Picradeniopsis (Compositae). –
Brittonia 25: 40-56.
Stuessy TF, Sang T, DeVore ML. 1996.
Phylogeny and biogeography of the subfamily Barnadesioideae with implications
for early evolution of Compositae. – In: Hind DJN, Beentje HJ (eds),
Compositae: systematics. Proceedings of the International Compositae
Conference, Kew, 1994, Royal Botanic Gardens, Kew, pp. 463-490.
Stuessy TF, Bloch C, Schneesweiss H, Rebernig
C, Villasenor J. 2008. Phylogeny and chromosome evolution in
Melampodium (Asteraceae).
– South Afr. J. Bot. 74: 379.
Stuessy TF, Urtubey E, Gruenstaeudl M. 2009.
Barnadesieae (Barnadesioideae). – In: Funk VA, Susanna A, Stuessy TF, Bayer
RJ (eds), Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 215-228.
Stuessy TF, Bloch C, Villaseñor JL, Rebernig
CA, Weiss-Schneeweiss H. 2011. Phylogenetic analyses of DNA sequences with
chromosomal and morphological data confirm and refine sectional and series
classification within Melampodium (Asteraceae, Millerieae). – Taxon
60: 436-449.
Suarez-Santiago VN, Salinas MJ, Garcia-Jacas
N, Soltis PS, Soltis DE, Blanca G. 2007. Reticulate evolution in the
Acrolophus subgroup (Centaurea L., Compositae) from the
western Mediterranean: origin and diversification of section
Willkommia Blanca. – Mol. Phylogen. Evol. 43: 156-172.
Subramanyam K. 1950a. Development of embryo
sac and endosperm in Stylidium tenellum. – Curr. Sci. 19: 294.
Subramanyam K. 1950b. An embryological study
of Levenhookia dubia. – Proc. Indian Natl. Inst. Sci., Sect. B, 16:
245-253.
Subramanyam K. 1951a. Interrelationships of
Campanulatae. – J. Mysore Univ., Sect. B, 12: 331-339.
Subramanyam K. 1951b. A morphological study
of Stylidium graminifolium Swartz. – Lloydia 14: 65-81.
Subramanyam K. 1951c. The origin and nature
of haustoria in Lobelia cardinalis L. – Bot. Gaz. 112: 319-322.
Subramanyam K. 1953. The nutritional
mechanism of embryo sac and embryo in the families Campanulaceae, Lobeliaceae, and Stylidiaceae. – J. Mysore Univ.,
sect. B, 13: 355-358.
Subramanyam K. 1970a. Comparative embryology
of angiosperms: Stylidiaceae.
– Bull. Natl. Sci. Acad. India 41: 317-320.
Subramanyam K. 1970b. Comparative embryology
of angiosperms: Crassulaceae, Campanulaceae, Sphenocleaceae, Pentaphragmataceae. – Bull.
Natl. Sci. Acad. India 41: 84-89, 306-312, 313-316, 321-324.
Sugiura T. 1942. Studies on the chromosome
numbers in Campanulaceae I.
Campanuloideae-Campanuleae. – Cytologia 12: 418-434.
Suh Y, Simpson BB. 1990. Phylogenetic
analysis of chloroplast DNA in North American Gutierrezia and related
genera. – Syst. Bot. 15: 660-670.
Sümbül HN, Göktürk RS, Düşen OD. 2003.
A new endemic species of Helichrysum Gaertn. (Asteraceae-Inuleae) from South
Anatolia. – Bot. J. Linn. Soc. 141: 251-254.
Sun M. 1986. Mixed mating systems and
gynodioecy in Hawaiian Bidens. – Ph.D. diss., University of British
Columbia, Vancouver, British Columbia.
Sundberg SD. 1985. Micromorphological
characters as generic markers in the Astereae. – Taxon 34: 31-37.
Sundberg SD. 1991. Infraspecific
classification of Chloracantha spinosa (Benth.) Nesom (Asteraceae-Astereae). – Phytologia
70: 382-391.
Sundberg SD, Cowan CP, Turner BL. 1986.
Chromosome counts of Latin American Compositae. – Amer. J. Bot. 73: 33-38.
Surina B, Rakič T, Stefanović V, Lakušić
D. 2009. One new species of the genus Edraianthus and a change in
taxonomic status for Edraianthus serpyllifolius f. pilosulus
(Campanulaceae) from the Balkan
Peninsula. – Syst. Bot. 34: 602-608.
Susanna A. 1996. Aneuploidy in the
Centaureinae (Compositae): is n = 7 the end of the series? – Taxon 45:
39-42.
Susanna A, Garcia-Jacas N. 2009. Cardueae
(Carduoideae). – In: Funk VA, Susanna A, Stuessy TF, Bayer RJ (eds),
Systematics, evolution, and biogeography of Compositae, International
Association for Plant Taxonomists, Wien, pp. 293-313.
Susanna A, Garcia-Jacas N, Soltis DE, Soltis
PS. 1995. Phylogenetic relationships in tribe Cardueae (Asteraceae) based on ITS sequences.
– Amer. J. Bot. 82: 1056-1068.
Susanna A, Garnatje T, Garcia-Jacas N. 1999.
Molecular phylogeny of Cheirolophus (Asteraceae: Cardueae-Centaureinae)
based on ITS sequences of nuclear ribosomal DNA. – Plant Syst. Evol. 214:
147-160.
Susanna A, Garnatje T, Garcia-Jacas N,
Vilatersana R. 2002. On the correct subtribal placement of the genera
Syreitschikovia and Nikitinia (Asteraceae, Cardueae): Carduinae or
Centaureinae? – Bot. J. Linn. Soc. 140: 313-319.
Susanna A, Garcia-Jacas N, Vilatersana R,
Garnatje T, Valles J, Ghaffari SM. 2003. New chromosome counts in the genus
Cousinia and the related genus Schmalhausenia (Asteraceae, Cardueae). – Bot. J.
Linn. Soc. 143: 411-418.
Susanna A, Garcia-Jacas N, Vilatersana R,
Garnatje T. 2003. Generic boundaries and evolution of characters in the
Arctium group: a nuclear and chloroplast DNA analysis. – Coll. Bot.
(Barcelona) 26: 102-118.
Susanna A, Garcia-Jacas N, Hidalgo O,
Vilatersana R, Garnatje T. 2006. The Cardueae (Compositae) revisited: insights
from ITS, trnL-trnF, and matK nuclear and
chloroplast DNA analysis. – Ann. Missouri Bot. Gard. 93: 150-171.
Susanna A, Galbany-Casals M, Romaschenko K,
Barres L, Martín J, Garcia-Jacas N. 2011. Lessons from Plectocephalus
(Compositae, Cardueae-Centaureinae): ITS disorientation in annuals and
Beringian dispersal as revealed by molecular analyses. – Ann. Bot. 108:
263-277.
Sütfeld R. 1982. Distribution of thiophene
derivatives in different organs of Tagetes patula seedlings grown
under various conditions. – Planta 156: 536-540.
Suyama C. 2001. A new naturalized plant,
Gymnocoronis spilanthoides DC. – J. Phytogeogr. Taxon. 49: 183-184.
[In Japanese]
Sventenius ER, Bramwell D. 1971.
Heywoodiella genus novum. – Acta Phytotaxon. Barcinon. 7: 1-8.
Swain T, Williams CA. 1977. Heliantheae –
chemical review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology
and chemistry of the Compositae II, Academic Press, London, pp. 673-697.
Swamy BGL. 1954. Morpho-taxonomical notes on
the Escallonioideae A. Nodal and petiolar vasculature. – J. Madras Univ.,
Sect. B, 24: 299-306.
Swelankomo N, Manning JC. 2014. The genus
Distephanus (Asteraceae:
Vernonieae) in southern Africa. – South Afr. J. Bot. 94: 238-248.
Swelankomo N, Mucina L, Herman PPJ. 2007.
Phenetic classification of cypselae in Ursinia (Anthemideae, Asteraceae). – South Afr. J. Bot.
73: 316.
Swelankomo N, Mucina L, Bellstedt DU. 2009. A
molecular phylogeny of the genus Ursinia (Anthemideae, Asteraceae) based on Internal
Transcribed Spacers of the nuclear ribosomal DNA. – South Afr. J. Bot. 75:
422.
Swelankomo N, Manning JC, Magee AR. 2016a.
The genus Gymnanthemum Cass. (Asteraceae: Vernonieae) in Southern
Africa. – South Afr. J. Bot. 102: 81-101.
Swelankomo N, Manning JC, Magee AR. 2016b.
The genus Hilliardiella (Asteraceae: Vernonieae) in southern
Africa. – South Afr. J. Bot. 106: 41-59.
Swenson U. 1995. Systematics of
Abrotanella, an amphipacific genus of Asteraceae (Senecioneae). – Plant
Syst. Evol. 197: 149-193.
Swenson U, Bremer K. 1994. The genus
Lagenocypsela (Asteraceae, Astereae) in New Guinea.
– Aust. Syst. Bot. 7: 265-273.
Swenson U, Bremer K. 1997a. Patterns of
floral evolution of four Asteraceae genera (Senecioneae,
Blennospermatinae) and the origin of white flowers in New Zealand. – Syst.
Biol. 46: 407-425.
Swenson U, Bremer K. 1997b. Pacific
biogeography of the Asteraceae
genus Abrotanella (Senecioneae, Blennospermatinae). – Syst. Bot. 22:
493-508.
Swenson U, Bremer K. 1999. On the
circumscription of the Blennospermatinae (Asteraceae, Senecioneae) based on
ndhF sequence data. – Taxon 48: 7-14.
Swenson U, Manns U. 2003. Phylogeny of
Pericallis (Asteraceae):
a total evidence approach reappraising the double origin of woodiness. –
Taxon 52: 533-546.
Swenson U, Nylinder S, Wagstaff SJ. 2012. Are
Asteraceae 1.5 billion years old?
A reply to Heads. – Syst. Biol. 61: 522-532.
Szelag Z. 2001. Hieracia balcanica I.
Hieracium ancevii (Asteraceae), eine neue Art aus
Bulgarien. – Feddes Repert. 112: 11-14.
Täckholm G. 1916. Zur Antipodenentwicklung
der Kompositengattungen Cosmidium und Cosmos. – Svensk Bot.
Tidskr. 10: 423-437.
Tadesse M. 1984a. The genus Bidens
(Compositae) in NE tropical Africa. – Symb. Bot. Ups. 24(1): 1-138.
Tadesse M. 1984b. Microlecane (Schl.
Bip.) Benth.: a congener of Bidens (Compositae-Heliantheae). – Nord.
J. Bot. 4: 737-746.
Tadesse M. 1990. Glossocardia and
Neuractis (Compositae), new records for Africa. – Kew Bull. 45:
141-145.
Tadesse M. 1992. New combinations and notes
in Compositae (Vernonieae, Inuleae s.l. and Heliantheae). – Comp. Newslett.
22: 11-17.
Tadesse M. 1993. Two new taxa of
Solanecio (Compositae: Senecioneae) from Ethiopia. – Kew Bull. 49:
137-141.
Tadesse M. 1995a. New species of
Iphiona and Pentanema (Compositae: Inuleae) from Somalia with
a note on P. indicum (L.) Ling in Africa. – Kew Bull. 50:
401-408.
Tadesse M. 1995b. Two new species of
Conyza (Compositae: Astereae) from NE & E tropical Africa. – Kew
Bull. 50: 627-632.
Tadesse M. 1997a. New species of
Vernonia (Compositae-Vernonieae) from Northeast Africa. – Kew Bull.
52: 593-600.
Tadesse M. 1997b. A revision of the genus
Echinops (Compositae-Cardueae) in tropical Africa. – Kew Bull. 52:
879-901.
Tadesse M, Beentje H. 2004. A synopsis and
new species of Emilia (Compositae-Senecioneae) in northeast tropical
Africa. – Kew Bull. 59: 469-482.
Tadesse M, Crawford DJ. 2014. The
phytomelanin layer in traditional members of Bidens and
Coreopsis and phylogeny of the Coreopsideae (Compositae). – Nord. J.
Bot. 32: 80-91.
Tadesse M, Reilly T. 1995. A contribution to
the study of Helichrysum (Compositae-Gnaphalieae). A revision of the
species of North-East tropical Africa. – In: Hind DJN, Jeffrey C, Pope GV
(eds), Advances in Compositae systematics, Royal Botanic Gardens, Kew, pp.
379-450.
Tadesse M, Crawford DJ, Smith EB. 1995.
Comparative capitular morphology and anatomy of Coreopsis L. and
Bidens L. (Compositae) including a review of generic boundaries. –
Brittonia 47: 61-91.
Tadesse M, Crawford DJ, Kim S-C. 2001. A
cladistic analyses of morphological features in Bidens L. and
Coreopsis L. (Asteraceae:
Heliantheae) with notes on generic delimitation and systematics. – In: Friis
I, Ryding O (eds) Biodiversity research in the Horn of Africa region. – Biol.
Skr., The Royal Danish Academy of Sciences and Letters, Copenhagen, pp.
85-102.
Tahara M. 1915. Cytological studies on
Chrysanthemum. – Bot. Mag. (Tokyo) 29: 48-50.
Talavera S. 1974. Contribución al studio
cariológico del género Cirsium en la Península Ibérica. –
Lagascalia 4: 285-296.
Talavera S, Valdés B. 1976. Revisión del
género Cirsium (Compositae) en la Península Ibérica. – Lagascalia
5: 127-223.
Talavera S, Devesa JA, Galliano EF. 1984.
Notas cariosistemáticas sobre plantas norteafricanas I. Compositae. –
Candollea 39: 271-280.
Talavera S, Arista M, Ortiz PL, Bastida F.
1994. Notas cariológicas sobre algunas Compuestas de Marruecos. – Acta Bot.
Malacitana 19: 97-101.
Taler I, Gailhoffer-Dengg E. 1972. Die
Feinstruktur der Zellkerneinschlusse von Campanula moesiaca, C.
persicifolia, and C. trachelium. – Phyton 14: 217-221.
Tamamshian SG. 1956. On the origin of the
pappus in the family Asteraceae
(Compositae). – Bot. Žurn. 41: 634-651.
Tamamshian SG. 1965. “Superevolutionary”
form of the calyx and its significance for phylogenetic problems in Asteraceae Link. – In: Problems in
plant phylogeny, Trudy Moscow Soc. Nat. 13: 161-174.
Tamanian K. 1999. Synopsis of the Caucasian
representatives of genus Cousinia (Asteraceae), Cardueae). – Feddes
Repert. 110: 73-79.
Tan K, Vural M. 2007. Centaurea
tchihatcheffii Fischer & C. A. Meyer (Asteraceae). – Plant Syst. Evol.
263: 203-207.
Tan K, Yıldız B. 1988. New
Asyneuma (Campanulaceae) taxa from Turkey.
– Willdenowia 18: 67-80.
Tank DC, Donoghue MJ. 2010. Phylogeny and
phylogenetic nomenclature of the Campanulidae based on an expanded sample of
genes and taxa. – Syst. Bot. 35: 425-441.
Tanowitz BD. 1982. Taxonomy of
Hemizonia sect. Madiomeris (Asteraceae: Madiinae). – Syst. Bot.
7: 314-339.
Tarasevich VF, Shresta KK. 1992.
Palynological data on the position of the genus Ostrowskia within the
family Campanulaceae. – Bot.
Žurn. 77: 27-30. [In Russian]
Tegel F. 2002. Die Testaepidermis der
Lactuceae (Asteraceae) – ihre
Diversität und systematische Bedeutung. – Ph.D. diss., Universität
München.
http://edoc.ub.uni-muenchen.de/archive/00000104/01/Tegel_Friedrich.pdf
Tellería MC. 2008. Taxonomic significance of
pollen types in the Guyana Highland-centred composite genera of Mutisioideae
(Asteraceae). – Bot. J. Linn.
Soc. 156: 327-340.
Tellería MC. 2012. Palynological survey of
the subtribe Elephantopinae (Asteraceae, Vernonieae). – Plant
Syst. Evol. 298: 1133-1139.
Tellería MC, Katinas L. 2004. A comparative
palynologic study of Chaetanthera (Asteraceae, Mutisieae) and allied
genera. – Syst. Bot. 29: 752-773.
Tellería MC, Katinas L. 2009. New insights
into the pollen morphology of the genus Mutisia (Asteraceae, Mutisieae). – Plant
Syst. Evol. 280: 229-241.
Tellería MC, Urtubey E, Katinas L. 2003.
Proustia and Lophopappus (Asteraceae, Mutisieae): generic and
subtribal relationships based on pollen morphology. – Rev. Paleobot. Palyn.
123: 237-246.
Telleria MC, Sancho G, Funk V, Ventosa I,
Roque N. 2013. Pollen morphology and its taxonomic significance in the tribe
Gochnatieae (Compositae, Gochnatioideae). – Plant Syst. Evol. 299:
935-948.
Tellería MC, Palazzesi L, Barreda V. 2015.
Evolutionary significance of exine ultrastructure in the subfamily
Barnadesioideae (Asteraceae) in
the light of molecular phylogenetics. – Rev. Palaeobot. Palynol. 221:
32-46.
Teppner H. 2001. The seedling of
Syneilesis (Asteraceae-Senecioneae), does it
possess cotyledons? – Fritschiana 26: 49-54.
Theisen I, Barthlott W. 1994.
Mikromorphologie der Epicuticularwachse und die Systematik der Gentianales, Rubiales, Dipsacales und Calycerales.
– Trop. Subtrop. Pflanzenwelt 89: 1-62.
Thellung A. 1916. Compositae. – In: Schinz
H (ed), Beiträge zur Kenntnis der afrikanischen Flora XXVII, Vierteljahrsschr.
Nat. Ges. Zürich 61: 431-461.
Thellung A. 1923. Compositae. – In: Schinz
H (ed), Beiträge zur Kenntnis der afrikanischen Flora XXXI, Vierteljahrsschr.
Nat. Ges. Zürich 68: 420-456.
Thiele E-M. 1988. Bau und Funktion des
Antheren-Griffel-Komplexes der Compositen. – Diss. Bot. 117: 1-169.
Thomas MM, Rudall PJ, Ellis AG, Savolainen V,
Glover BJ. 2009. Development of a complex floral trait: the
pollinator-attracting petal spots of the beetle daisy, Gorteria
diffusa (Asteraceae). –
Amer. J. Bot. 96: 2184-2196.
Thompson SW, Lammers TG. 1997. Phenetic
analysis of morphological variation in the Lobelia cardinalis complex
(Campanulaceae: Lobelioideae).
– Syst. Bot. 22: 315-331.
Thornton-Wood SP. 1993. A taxonomical study
of the yellow-flowering species of Achillea (sect. Filipendulinae (D.)
Boiss.) (Asteraceae) in the Balkan
Peninsula. – Ph.D. diss., University of Reading, England.
Thulin M. 1974. Gunillaea and
Namacodon. Two new genera of Campanulaceae in Africa. – Bot.
Not. 127: 165-182.
Thulin M. 1975. The genus
Wahlenbergia s. lat. (Campanulaceae) in Tropical Africa
and Madagascar. – Symb. Bot. Ups. 21(1): 1-223.
Thulin M. 1976. Campanula keniensis
Thulin sp. nov., and notes on allied species. – Bot. Not. 128: 350-356.
Thulin M. 1976. Campanulaceae. – In: Polhill RM
(ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, pp. 1-39.
Thulin M. 1977. Campanulaceae. – In: Flore
d’Afrique Centrale, Jardin Botanique National de Belgique, Bruxelles, pp.
1-50.
Thulin M. 1978. Cyphia (Lobeliaceae)
in tropical Africa. – Bot. Not. 131: 455-471.
Thulin M. 1979. Monopsis
(Lobeliaceae) in tropical Africa. – Bot. Not. 132: 131-137.
Thulin M. 1983a. Some tropical African
Lobeliaceae. Chromosome numbers, new taxa, and comments on taxonomy and
nomenclature. – Nord. J. Bot. 3: 371-382.
Thulin M. 1983b. 99. Campanulaceae. – In: Launert E
(ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing Committee, London,
pp. 87-114.
Thulin M. 1983c. 101. Lobeliaceae. – In:
Launert E (ed), Flora Zambesiaca 7 (Part 1), Flora Zambesiaca Managing
Committee, London, pp. 116-157.
Thulin M. 1984. Lobeliaceae. – In: Polhill
RM (ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The
Netherlands, pp. 1-59.
Thulin M. 1987. New species of
Wahlenbergia (Campanulaceae) from Africa. –
Nord. J. Bot. 7: 261-265.
Thulin M. 2001. Pentzia (Asteraceae-Anthemideae) in the Horn
of Africa region. – Nord. J. Bot. 21: 249-252.
Tiagi B, Taimni S. 1960. Embryo sac
development in Vernonia cinerascens and seed development in V.
cinerea. – Curr. Sci. 29: 406.
Tiagi B, Taimni S. 1963. Floral morphology
and embryology of Vernonia cinerascens and V. cinerea. –
Agra Univ. J. Res. (Sci.) 12: 123-137.
Timme RE, Simpson BB, Linder CR. 2007.
High-resolution phylogeny for Helianthus (Asteraceae) using the 18S-26S
ribosomal DNA external transcribed spacer. – Amer. J. Bot. 94: 1837-1852.
Tippery NP, Les DH. 2008. Phylogenetic
analysis of the internal transcribed spacer (ITS) region in Menyanthaceae using predicted
secondary structure. – Mol. Phylogen. Evol. 49: 526-537.
Tippery NP, Les DH. 2009. A new genus and new
combinations in Australian Villarsia (Menyanthaceae). – Novon 19:
406-413.
Tippery NP, Les DH, Padgett DJ, Jacobs SWL.
2008. Generic circumscription in Menyanthaceae: a phylogenetic
evaluation. – Syst. Bot. 33: 598-612.
Tippery NP, Les DH, Regalado Jr JC, Averyanov
LV, Vu NL, Raven PH. 2009. Transfer of Villarsia cambodiana to
Nymphoides (Menyanthaceae). – Syst. Bot. 34:
818-823.
Tippery NP, Schilling EE, Panero JL, Les DH,
Williams CS. 2014. Independent origins of aquatic Eupatorieae (Asteraceae). – Syst. Bot. 39:
1217-1225.
Tirel C. 1996. Reétablissement de
Periomphale Baill. (Alseuosmiaceae), genre endémique
de Nouvelle Calédonie. – Bull. Mus. Natl. Hist. Nat. Paris, sér IV, sect.
B, Adansonia 18: 155-160.
Tirel C, Jérémie J. 1996. Alseuosmiaceae. – In: Morat P
(ed), Flore de la Nouvelle-Calédonie et Dépendances 20, Muséum National
d’Histoire Naturelle, Paris, pp. 100-106.
Tjon Sie Fat L. 1978. Contribution to the
knowledge of cyanogenesis in angiosperms 2. Communication: cyanogenesis in Campanulaceae. – Proc. Kon.
Nederl. Akad. Wetensch., ser. C., 81: 126-131.
Tobe H, Morin NR. 1996. Embryology and
circumscription of Campanulaceae and Campanulales: a review of the
literature. – Intern. J. Plant Res. 109: 425-435.
Tomb AS. 1972. Re-establishment of the genus
Prenanthella Rydb. (Compositae: Cichorieae). – Brittonia 34:
223-228.
Tomb AS. 1976. Pollen morphology in tribe
Lactuceae (Compositae). – Grana 15: 79-89.
Tomb AS. 1977. Lactuceae – systematic
review. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae, Academic Press, London, pp. 1067-1079.
Tomb AS. 1980. Taxonomy of
Lygodesmia (Asteraceae).
– Syst. Bot. Monogr. 1: 1-51.
Tomb AS, Larson DA, Skvarla JJ. 1974. Pollen
morphology and detailed structure of family Compositae, tribe Cichorieae I.
Subtribe Stephanomeriinae. – Amer. J. Bot. 61: 486-498.
Tongiorgi E. 1935. Un primo contributo alla
cariologia delle Inuleae. – Nuovo Giorn. Bot. Ital., n. s., 42: 261-262.
Tonjan ZR. 1968. The chromosome numbers of
some species of the genus Centaurea. – Biol. Žurn. Armenii 21:
86-96. [In Russian]
Torices R. 2010. Adding time-calibrated
branch lengths to the Asteraceae
supertree. – J. Syst. Evol. 48: 271-278.
Torices R, Anderberg AA. 2009. Phylogenetic
analysis of sexual systems in Inuleae (Asteraceae) – Amer. J. Bot. 96:
1011-1019.
Torrell M, Garcia-Jacas N, Susanna A, Vallès
J. 1999. Phylogeny in Artemisia (Asteraceae, Anthemideae) inferred
from nuclear ribosomal DNA (ITS) sequences. – Taxon 48: 721-736.
Torrell M, Vallès J, Garcia-Jacas N,
Mozaffarian V, Gabrielian E. 2001. New or rare chromosome counts in the genus
Artemisia L. (Asteraceae,
Anthemideae) from Armenia and Iran. – Bot. J. Linn. Soc. 135: 51-60.
Tortosa RD, Bartoli A. 2002. Two new species
of Haplopappus (Astereae, Asteraceae) from Mendoza, Argentina.
– Brittonia 54: 50-53.
Towers CHN, Champagne D. 1988. Medicinal
phytochemistry of the Compositae: the activities of selected acetylenes and
their sulfur derivatives. – In: Lam J, Breteler H, Arnason JT, Hansen L
(eds), Chemistry and biology of naturally-occurring acetylenes and related
compounds (NOARC), Bioactive molecules, Elsevier, Amsterdam, vol. 7, pp.
139-150.
Townsend CC. 1988. Contributions to the Flora
of Iraq XV. Concerning two species of Iraqi Echinops (Compositae). –
Kew Bull. 43: 111-114.
Trach NV, Hoffmann MH, Röser M, Korobkov AA,
Hagen KB von. 2008. Parallel evolutionary patterns in multiple lineages of
Arctic Artemisia L. (Asteraceae). – Evolution 62:
184-198.
Tremetsberger K, Weiss-Schneeweiss H, Stuessy
T, Samuel R, Kadlec G, Ortiz MA, Talavera S. 2005. Nuclear ribosomal DNA and
karyotypes indicate a NW African origin of South American Hypochaeris
(Asteraceae, Cichorieae). – Mol.
Phylogen. Evol. 35: 102-116.
Tremetsberger K, Stuessy TF, Kadlec G,
Urtubey E, Baeza CM, Beck SG, Valdebenito HA, Ruas C de Fatima, Matzenbacher
NI. 2006. AFLP phylogeny of South American species of Hypochaeris (Asteraceae, Lactuceae). – Syst.
Bot. 31: 610-626.
Tremetsberger K, Gemeinholzer B, Zetzsche H,
Blackmore S, Kilian N, Talavera S. 2012. Divergence time estimation in
Cichorieae (Asteraceae) using a
fossil-calibrated relaxed molecular clock. – Organ. Divers. Evol. 12:
1-16.
Trisos CH, Verboom GA, Bergh NG. 2008.
Phylogeny of the daisy genera Ifloga and Trichogyne (Asteraceae: Gnaphalieae) and
evaluation of the link between annuality and aridity in the succulent karoo.
– South Afr. J. Bot. 74: 379.
Troiani HO, Steibel PE. 2006. Senecio
chipauquilensis (Asteraceae,
Senecioneae), a new species from the Meseta de Somuncurá (Patagonia),
Argentina. – Brittonia 58: 285-287.
Tugay O, Uysal T, Ertugrul K. 2009.
Psephellus yusufeliensis sp. nov. from north-east Anatolia, Turkey.
– Nord. J. Bot. 27: 134-137.
Tuisl G. 1968. Der Verwandtschaftskreis der
Gattung Lactuca L. im iranischen Hochland. Vorarbeiten zur Flora
Iranica Nr. 16. – Ann. Naturhist. Mus. Wien 72: 587-638.
Turland NJ. 2008. Anthemis
samariensis (Asteraceae,
Anthemideae), a new species from the mountains of W Kriti (Greece). –
Willdenowia 38: 61-69.
Turner BL. 1956. A cytotaxonomic study of
Hymenopappus (Compositae). – Rhodora 58: 163-186, 208-242, 250-269,
295-308.
Turner BL. 1962. Taxonomy of
Hymenothrix (Helenieae, Compositae). – Brittonia 14: 101-120.
Turner BL. 1963. Taxonomy of
Florestina (Helenieae, Compositae). – Brittonia 15: 27-46.
Turner BL. 1964. Chromosome numbers in the
Compositae VIII. Mexican and Central American species. – Southwest. Natur. 9:
27-39.
Turner BL. 1966. Taxonomy of
Oxypappus (Compositae). – Sida 2: 431-433.
Turner BL. 1970. Chromosome numbers in the
Compositae XII. Australian species. – Amer. J. Bot. 5: 382-389.
Turner BL. 1973a. Two new gypsophilous
species of Machaeranthera (Asteraceae: Astereae) from
north-central Mexico. – Phytologia 26: 116-120.
Turner BL. 1973b. Machaeranthera
restiformis (Asteraceae), a
bizarre new gypsophile from north-central Mexico. – Amer. J. Bot. 60:
836-838.
Turner BL. 1977a. Fossil history and
geography. – In: Heywood VH, Harborne JB, Turner BL (eds), The biology and
chemistry of the Compositae, Academic Press, London, pp. 21-39.
Turner BL. 1977b. Summary of the biology and
chemistry of the Compositae. – In: Heywood VH, Harborne JB, Turner BL (eds),
The biology and chemistry of the Compositae, Academic Press, London, pp.
1105-1118.
Turner BL. 1980. La taxonomía del género
Aphanactis (Asteraceae-Heliantheae). – Bol.
Soc. Argent. Bot. 19: 33-44.
Turner BL. 1981. New species and combinations
in Vernonia sections Leiboldia and Lepidoma (Asteraceae), with a revisional
conspectus of the groups. – Brittonia 33: 401-412.
Turner BL. 1984. Taxonomy of the genus
Aphanostephus (Asteraceae-Astereae). – Phytologia
56: 81-101.
Turner BL. 1987a. Taxonomic study of
Machaeranthera, sections Machaeranthera and
Hesperastrum (Asteraceae). – Phytologia 62:
207-266.
Turner BL. 1987b. Submergence of the genera
Caterothamnus and Oaxacania into Hofmeisteria
(Eupatorieae, Asteraceae). –
Phytologia 63: 415-416.
Turner BL. 1987c. Taxonomy of
Carphochaete (Asteraceae-Eupatorieae). –
Phytologia 64: 145-162.
Turner BL. 1988a. Submergence of the genera
Asanthus and Discritogyne within Steviopsis (Asteraceae, Eupatorieae), including
new combinations. – Phytologia 64: 259-262.
Turner BL. 1988b. Taxonomic study of
Chrysanthellum (Asteraceae, Coreopsideae). –
Phytologia 64: 410-444.
Turner BL. 1989. Revisionary treatment of the
genus Sinclairia, including Liabellum (Asteraceae, Liabeae). – Phytologia
67: 168-206.
Turner BL. 1990a. Taxonomy of
Varilla (Asteraceae:
Heliantheae). – Phytologia 68: 4-13.
Turner BL. 1990b. A new species of
Steviopsis (Asteraceae:
Eupatorieae) from Nuevo Leon, Mexico. – Phytologia 68: 410-412.
Turner BL. 1990c. A reevaluation of the genus
Alepidocline (Asteraceae,
Heliantheae, Galinsoginae) and description of a new species from Oaxaca,
Mexico. – Phytologia 69: 387-392.
Turner BL. 1991a. Recension of the
Cronquistianthus group of Eupatorium (Asteraceae). – Phytologia 70:
158-177.
Turner BL. 1991b. Brickellia
sonorana (Asteraceae), a new
species from México belonging to the subgenus Phanerostylis. –
Phytologia 71: 51-56.
Turner BL. 1991c. Nesomia chiapensis
(Asteraceae-Eupatorieae), a new
genus and species from Mexico. – Phytologia 71: 208-211.
Turner BL. 1994. Taxonomic status of
Brickelliastrum villarrealii R. M. King & H. Robins. (Asteraceae, Eupatorieae). –
Phytologia 76: 389-390.
Turner BL. 1995. Synopsis of
Ageratella (Asteraceae,
Eupatorieae). – Phytologia 78: 204-208.
Turner BL. 1996. The genus Liabum
(Asteraceae: Liabeae) in the
Dominican Republic and Haiti. – Phytologia 80: 115-117.
Turner BL. 1997. Eupatorieae. – In: Turner
BL (ed), The Compositae of Mexico. A systematic account of the family Asteraceae 1, Phytologia Mem. 11:
i-iv, 1-272.
Turner BL. 2007. The comps of Mexico: a
systematic account of the family Asteraceae 8, Liabeae and Vernonieae.
– Phytologia Mem. 12: 1-144.
Turner BL. 2009. Three new species of
Koanophyllon (Asteraceae:
Eupatorieae) from Mexico. – Phytologia 91: 312-324.
Turner BL. 2010. Recension of the Mexican
Amauriopsis (Asteraceae:
Bahieae). – Phytoneuron 2010-10: 1-7.
Turner BL. 2013. Five new species of
Ageratina (Asteraceae:
Eupatorieae) from Oaxaca, Mexico. – Phytologia 95: 141-150.
Turner BL. 2014a. Three new species of
Stevia (Asteraceae:
Eupatorieae) from northern Coahuila. – Phytologia 96: 117-123.
Turner BL. 2014b. The genus Asanthus
(Asteraceae: Eupatorieae)
revisited. – Phytologia 96: 195-198.
Turner BL. 2014c. Texas taxa of the genus
Belandiera (Asteraceae:
Heliantheae). – Phytologia 96: 235-240.
Turner BL. 2015. Three new species of
Stevia (Asteraceae:
Eupatorieae) from northern Mexico. – Phytologia 97: 25-31.
Turner BL, Flyr D. 1966. Chromosome numbers
in the Compositae X. North American species. – Amer. J. Bot. 53: 24-33.
Turner BL, Horn D. 1964. Taxonomy of
Machaeranthera sect. Psilactis (Compositae-Astereae). –
Brittonia 16: 316-331.
Turner BL, Irwin HS. 1960. Chromosome numbers
in the Compositae II. Meiotic counts for fourteen species of Brazilian
Compositae. – Rhodora 62: 122-126.
Turner BL, King RM. 1962. A cytotaxonomic
survey of Melampodium (Compositae-Heliantheae). – Amer. J. Bot. 49:
263-269.
Turner BL, King RM. 1964. Chromosome numbers
in the Compositae VIII. Mexican and Central American species. – Southw.
Natur. 9: 27-39.
Turner BL, Lewis WH. 1965. Chromosome numbers
in the Compositae IX. African species. – J. South Afr. Bot. 31: 207-213.
Turner BL, Morris MI. 1976. Systematics of
Palafoxia (Asteraceae:
Helenieae). – Rhodora 78: 567-628.
Turner BL, Powell AM. 1977. Helenieae –
systematic review. – In: Heywood VH, Harborne JB, Turner BL (eds), The
biology and chemistry of the Compositae, Academic Press, London, pp.
699-737.
Turner BL, Sundberg SD. 1986. Systematic
study of Osbertia (Asteraceae-Astereae). – Plant Syst.
Evol. 151: 229-239.
Turner BL, Zippin D. 1992. Taxonomic study of
Venegasia (Asteraceae:
Helenieae). – Sida 15: 223-229.
Turner BL, Ellison WL, King RM. 1961.
Chromosome numbers in the Compositae IV. North American species, with phyletic
interpretations. – Amer. J. Bot. 48: 216-223.
Turner BL, Beaman JH, Rock HFL. 1961.
Chromosome numbers in the Compositae V. Mexican and Guatemalan species. –
Rhodora 63: 121-129.
Turner BL, Powell AM, King RM. 1962.
Chromosome numbers in the Compositae VI. Additional Mexican and Guatemala
species. – Rhodora 64: 251-271.
Turner BL, Powell AM, Cuatrecasas J. 1967.
Chromosome numbers in the Compositae XI. Peruvian species. – Ann. Missouri
Bot. Gard. 54: 172-177.
Turner BL, Bacon J, Urbatsch L, Simpson B.
1979. Chromosome numbers in South American Compositae. – Amer. J. Bot. 66:
173-178.
Turner BL, Kim KJ. Norris J. 1991. Taxonomic
status of Barroetea glutinosa (Asteraceae, Eupatorieae) and its
allies: morphological evidence for the transfer of Barroetea to
Brickellia. – Phytologia 71: 38-50.
Turner MW. 1993. Systematic study of the
genus Baileya (Asteraceae: Helenieae). – Sida 15:
491-508.
Tyrl RJ. 1975. Origin and distribution of
polyploidy Achillea (Compositae) in western North America. –
Brittonia 27: 187-196.
Tzvelev N. 1959. Notulae criticae de
sectionibus nonnullis generis Centaureeae L. – Bot. Mater. Gerb. Bot. Inst.
Komarova Akad. Nauk SSSR 19: 409-441. [In Russian]
Ueno J. 1972. The fine structure of pollen
surface III: Gazania and Stokesia. – Rep. Fac. Sci.
Shizuoka Univ. 7:103-116.
Uesugi R, Goka K, Nishihiro J, Washitani I.
2004. Allozyme polymorphism and conservation of the Lake Kasumigaura population
of Nymphoides peltata. – Aquatic Bot. 79: 203-210.
Uexkull-Gyllenband M von. 1901. Phylogenie
der Blütenformen und der Geschlechter verteilung bei den Compositen. – Bibl.
Bot. 52: 1-80.
Uhlemann I. 2002. A new species of the genus
Taraxacum F. H. Wigg. (Asteraceae, Lactuceae) from South
America. – Feddes Repert. 113: 329-334.
Uhlemann I, Ritz CM, Panailillo P. 2009.
Relationships in Taraxacum section Arctica s.l. (Asteraceae, Cichorieae) and allies
based on nrITS. – Feddes Repert. 120: 35-47.
Uitz H. 1970. Cytologische und
bestäubungsexperimentelle Beiträge zur Verwandtschaft und Evolution der
Anthemideae (Asteraceae). –
Thesis, Universität Graz, Austria.
Unal O, Gokturk RS. 2003. A new species of
Scorzonera L. (Asteraceae) from South Anatolia,
Turkey. – Bot. J. Linn. Soc. 142: 465-468.
Urban I. 1881. Die Bestäubungseinrichtungen
bei den Lobeliaceen nebst einer Monographie der afrikanischen
Lobeliaceen-Gattung Monopsis. – Jahrb. Königl. Bot. Gart. Berlin 1:
260-277.
Urbanska-Worytkiewicz K. 1967. Cytological
investigations in Antennaria Gaertn. from North Scandinavia. – Acta
Borealia, A 22: 1-14.
Urbatsch LE, Jansen RK. 1995. Phylogenetic
affinities among and within the coneflower genera (Asteraceae, Heliantheae): a
chloroplast DNA analysis. – Syst. Bot. 20: 28-39.
Urbatsch LE, Roberts RP. 2004. New
combinations in the genus Gundlachia and four new genera of Astereae
(Asteraceae) from northern Mexico
and the southern United States. – Sida 21: 243-257.
Urbatsch LE, Zlotsky A, Pruski JF. 1986.
Revision of Calea sect. Lemmatium (Asteraceae: Heliantheae) from Brazil.
– Syst. Bot. 11: 501-514.
Urbatsch LE, Baldwin BG, Donoghue MJ. 2000.
Phylogeny of the coneflowers and relatives (Heliantheae: Asteraceae) based on nuclear rDNA
internal transcribed spacer (ITS) sequences and chloroplast DNA restriction
site data. – Syst. Bot. 25: 539-565.
Urbatsch LE, Roberts RP, Karaman V. 2003.
Phylogenetic evaluation of Xylothamia, Gundlachia, and
related genera (Asteraceae,
Astereae) based on ETS and ITS nrDNA sequence data. – Amer. J. Bot. 90:
634-649.
Uribe-Convers AS, Carlsen MM, Lagomarsino LP,
Muchhala N. 2017. Phylogenetic relationships of Burmeistera (Campanulaceae: Lobelioideae):
combining whole plastome with targeted loci data in a recent radiation. –
Mol. Phylogen. Evol. 107: 551-563.
Urtubey E, Stuessy TF. 2001. New hypotheses
of phylogenetic relationships in Barnadesioideae (Asteraceae) based on morphology. –
Taxon 50: 1043-1066.
Urtubey E, Tellería MC. 1998. Pollen
morphology of the subfamily Barnadesioideae (Asteraceae) and its phylogenetic and
taxonomic significance. – Rev. Palaeobot. Palynol. 104: 19-37.
Urtubey E, Stuessy TF, Tremetsberger K. 2009.
Systematics of the South American Hypochaeris sessiliflora complex (Asteraceae, Cichorieae). – Ann.
Missouri Bot. Gard. 96: 685-714.
Urtubey E, López A, Chemisquy MA, Anderberg
AA, Baewza CM, Bayón ND, Deble LP, Moreira-Muñoz A, Nesom GL, Alford MH,
Salomón L, Freire SE. 2016. New circumscription of the genus
Gamochaeta (Asteraceae,
Gnaphalieae) inferred from nuclear and plastid DNA sequences. – Plant Syst.
Evol. 302: 1047-1066.
Utelli A-B, Huber W (†). 1996. The
hypothesis of introgression in Alpine Erigeron (Asteraceae): a multivariate
morphological approach. – Willdenowia 25: 443-454.
Uzunhisarcikli ME, Dogan E, Duman H. 2007. A
new species of Centaurea L. (Cardueae: Asteraceae) from Turkey. – Bot. J.
Linn. Soc. 153: 61-66.
Vaezi J, Brouillet L. 2009. Phylogenetic
relationships among diploid species of Symphyotrichum (Asteraceae: Astereae) based on two
nuclear markers, ITS and GAPDH. – Mol. Phylogen. Evol. 51: 540-553.
Vaidya PB. 1961. Male and female gametophytes
of Acanthospermum hispidum D. C. – J. Biol. Sci. 4: 11-15.
Valadon LRG. 1977. Arctot[id]eae and
Calenduleae – chemical review. – In: Heywood VH, Harborne JB, Turner BL
(eds), The biology and chemistry of the Compositae 2, Academic Press, London,
pp. 989-998.
Valant-Vetschera KM. 1982. Flavonoid pattern
and systematics of the genus Leucocyclus. – Phytochemistry 21:
1067-1069.
Valant-Vetschera KM. 1987. Flavonoid
glycoside accumulation trends of Achillea nobilis and related species.
– Biochem. Syst. Ecol. 15: 45-52.
Valant-Vetschera KM. 1999. On the identity of
five species of Achillea sect. Millefolium subsect.
Filipendulinae (Compositae, Anthemideae). – Willdenowia 29:
141-146.
Valant-Vetschera KM, Kästner A. 1998. New
combinations and synonyms in Achillea sect. Santolinoidea
from North Africa and Turkey. – Feddes Repert. 109: 501-508.
Valant-Vetschera KM, Kästner A. 2000.
Character analysis in Achillea sect. Santolinoidea
(Compositae-Anthemideae) I. Leaf and floral morphology. – Edinburgh J. Bot.
57: 189-208.
Valant-Vetschera KM, Wollenweber E. 1989.
Flavonoid aglycones in the leaf exudate of the genus Leucocyclus. –
Zeitschr. Naturforsch. 44: 323-324.
Valant-Vetschera KM, Wollenweber E. 1996.
Comparative analysis of leaf exudate flavonoids in Achillea subsect.
Filipendulinae. – Biochem. Syst. Ecol. 24: 435-446.
Valdebenito H, Stuessy TF, Crawford DJ, Silva
O M. 1992. Evolution of Erigeron (Compositae) in the Juan Fernandez
Islands, Chile. – Syst. Bot. 17: 470-480.
Valdés Bermejo E, Agudo Mata MP. 1983.
Estudios cariológicos en especies ibéricas del género Centaurea L.
(Compositae) I. – An. Jard. Bot. Madrid 40: 119-142.
Vallès J, Garnatje T. 2005.
Artemisia and its allies: genome organization and evolution and their
biosystematic, taxonomic, and phylogenetic implications in the Artemisiinae and
related subtribes (Asteraceae,
Anthemidae). – In: Sharma AK, Sharma A (eds), Plant genome: biodiversity and
evolution 1 B. Phanerogams (higher groups), Scientific Publ., Enfield, New
Hampshire, pp. 255-285.
Vallès J, Torrell M, Garcia-Jacas N. 2001.
New or rare chromosome counts in Artemisia L. (Asteraceae, Anthemideae) and related
genera from Kazakhstan. – Bot. J. Linn. Soc. 137: 399-407.
Vallès J, Torrell M, Garnatje T,
Garcia-Jacas N, Vilatersana R, Susanna A. 2003. The genus Artemisia
and its allies: phylogeny of the subtribe Artemisiinae (Asteraceae, Anthemideae) based on
nucleotide sequences of nuclear ribosomal DNA internal transcribed spacers
(ITS). – Plant Biol. 5: 274-284.
Vallès J, Torrell M, Garcia-Jacas N,
Kapustina LA. 2001. New or rare chromosome counts in the genera
Artemisia L. and Mausolea Bunge (Asteraceae, Anthemideae) from
Uzbekistan. – Bot. J. Linn. Soc. 135: 391-400.
Vallès J, Garnatje T, Garcia S, Sanz M,
Korobkov A. 2005. Chromosome numbers in the tribes Anthemideae and Inuleae (Asteraceae). – Bot. J. Linn. Soc.
148: 77-85.
Vallès J, Pellicer J, Sánchez-Jiménez I,
Hidalgo O, Vitales D,
Garcia S, Martín J, Garnatje T. 2012. Polyploidy and other changes at
chromosomal level and in genome size: its role in systematics and evolution
exemplified by some genera of Anthemideae and Cardueae (Asteraceae). – Taxon 61:
841-851.
Vanijajiva O, Kadereit JW. 2008. A revision
of Cissampelopsis (Asteraceae: Senecioneae). – Kew
Bull. 63: 213-226.
Vanijajiva O, Kadereit JW. 2011. A revision
of Gynura (Asteraceae:
Senecioneae). – J. Syst. Evol. 49: 285-314.
Vargas O M. 2011. A nomenclator of
Diplostephium (Asteraceae: Astereae): a list of
species with their synonyms and distribution by country. – Lundellia 14:
32-51.
Vargas OM. 2018. Reinstatement of the genus
Piofontia: a phylogenomic-based study reveals the biphyletic nature of
Diplostephium (Asteraceae: Astereae). – Syst. Bot.
43: 485-496.
Vargas OM, Madriñán S. 2012. Preliminary
phylogeny of Diplostephium (Asteraceae): speciation rate and
character evolution. – Lundellia 15: 1-15.
Vargas OM, Ortiz EM, Simpson BB. 2017.
Conflicting phylogenomic signals reveal a pattern of reticulate evolution in a
recent high-Andean diversification (Asteraceae: Astereae:
Diplostephium). – New Phytol. 214: 1736-1750.
Varma PG, Vijayavalli B. 2002. Studies in the
pollen morphology of Inuleae and Heliantheae of Asteraceae. – J. Palynol. 34:
51-83.
Vasilevskaya VK, Shulkina TV. 1976.
Morphological and anatomical structure of the arborescent plant Azorina
vidalii. – Trudy Moskovsk. Obšč. Isp. Prir. 42: 131-140. [In
Russian]
Vasudevan Nair R. 1975. Heterostyly and
breeding system of Nymphoides cristatum (Roxb.) O. Kuntze. – J.
Bombay Nat. Hist. Soc. 72: 677-682.
Veldkamp JF, Kreffer LA. 1991. Notes on
Southeast Asian and Australian Coreopsidinae (Asteraceae). – Blumea 35:
459-482.
Velez MC. 1981. Karpologische Untersuchungen
an amerikanischen Astereae (Compositae). – Mitt. Bot. Staatssamml. München
17: 1-170.
Ventosa-Rodriguez I, Herrera-Oliver PP.
2011a. Do the Antillean species of Gochnatia Kunth (Asteraceae) truly belong in that
genus? A phylogenetic analysis based on morphological characters. –
Compositae Newsletter 49: 8-22.
Ventosa-Rodriguez I, Herrera-Oliver PP.
2011b. Restoration of the name Anastraphia to define the species in
the section Anastraphioides of Gochnatia (Gochnatioideae, Asteraceae). – Compositae
Newsletter 49: 23-37.
Vermeer J, Peterson RL. 1979a. Glandular
trichomes on the inflorescence of Chrysanthemum morifolium cv.
Dramatic (Compositae) I. Development and morphology. – Can. J. Bot. 57:
705-713.
Vermeer J, Peterson RL. 1979b. Glandular
trichomes on the inflorescence of Chrysanthemum morifolium cv.
Dramatic (Compositae) II. Ultrastructure and histochemistry. – Can. J. Bot.
57: 714-729.
Vezey EL, Watson LE, Skvarla JJ, Estes JR.
1994. Plesiomorphic and apomorphic pollen structure characteristics of
Anthemideae (Asteroideae: Asteraceae). – Amer. J. Bot. 81:
648-657.
Vierhapper F. 1908. Monographie der alpinen
Erigeron-Arten Europas und Vorderasiens. – Beih. Bot. Centralbl 19:
385-560.
Vijayaraghavan MR, Malik U. 1972. Morphology
and embryology of Scaevola frutescens K. and affinities of the family
Goodeniaceae. – Bot. Not. 125:
241-254.
Vijverberg K, Mes RHM, Bachmann K. 1999.
Chloroplast DNA evidence for the evolution of Microseris (Asteraceae) in Australia and New
Zealand after long-distance dispersal from western North America. – Amer. J.
Bot. 86: 1448-1463.
Vijverberg K, Kuperus P, Breeuwer JAJ,
Bachmann K. 2000. Incipient adaptive radiation of New Zealand and Australian
Microseris (Asteraceae):
an amplified fragment length polymorphism (AFLP) study. – J. Evol. Biol. 13:
997-1008.
Vilatersana R, Susanna A, Garcia-Jacas N,
Garnatje T. 2000. Karyology, generic delineation and dysploidy in the genera
Carduncellus, Carthamus and Phonus (Asteraceae). – Bot. J. Linn. Soc.
134: 425-438.
Vilatersana R, Susanna A, Garcia-Jacas N,
Garnatje T. 2000. Generic delimitation and phylogeny of the
Carduncellus-Carthamus complex (Asteraceae) based on ITS sequences.
– Plant Syst. Evol. 221: 89-105.
Vilatersana R, Garnatje T, Susanna A,
Garcia-Jacas N. 2005. Taxonomic problems in Carthamus (Asteraceae): RAPD markers and
sectional classification. – Bot. J. Linn. Soc. 147: 375-383.
Vilatersana R, Susanna A, Brochmann C. 2007.
Genetic variation in Femeniasia (Compositae, Cardueae), an endemic and
endangered monotypic genus from the Balearic Islands (Spain). – Bot. J. Linn.
Soc. 153: 97-107.
Vilatersana R, Garcia-Jacas N, Garnatje T,
Molero J, Sonnante G, Susanna A. 2010. Molecular phylogeny of the genus
Ptilostemon (Compositae: Cardueae) and its relationships with
Cynara and Lamyropsis. – Syst. Bot. 35: 907-917.
Villard M. 1970. Contribution à l’étude
cytotaxonomique et cytogenetique du genre Leucanthemum Adans. em.
Briq. et Cav. – Bull. Soc. Bot. Suisse 80: 96-188.
Villasenor JL, Strother JL. 1989.
Tuxtla, a new genus for Zexmenia pittieri (Compositae:
heliantheae). – Syst. Bot. 14: 529-540.
Villodre JM, Garcia-Jacas N. 2000. Pollen
studies in subtribe Centaureinae (Asteraceae): the Jacea group
analysed with electron microscopy. – Bot. J. Linn. Soc. 133: 473-484.
Vincent PLD. 1996. Progress on clarifying the
generic concept of Senecio based on an extensive world-wide sample of
taxa. – In: Hind DJN, Beentje HJ (eds), Compositae: systematics. Proceedings
of the International Compositae Conference, Kew, 1994, I, Royal Botanic
Gardens, Kew, pp. 597-611.
Vincent PLD, Getliffe FM. 1988. The
endothecium in Senecio (Compositae). – Bot. J. Linn. Soc. 97:
63-71.
Vincent PLD, Norris FMG. 1989. A SEM study of
the external pollen morphology in Senecio and some related genera in
the subtribe Senecioninae (Asteraceae: Senecioneae). – S.-Afr.
Tydskr. Plantk. 55: 304-309.
Vitales D, Garnatje T,
Pellicer J, Vallès J, Santos-Guerra A, Sanmartín I. 2014. The explosive
radiation of Cheirolophus (Asteraceae, Cardueae) in Macaronesia.
– BMC Evol. Biol. 14: 1-29.
Vladimirov V. 2003. A new diploid
Hieracium (Asteraceae:
Lactuceae) from Bulgaria. – Bot. J. Linn. Soc. 143: 213-218.
Vogel S. 1998. Remarkable nectaries:
structure, ecology, organophyletic perspectives IV. Miscellaneous cases. –
Flora 193: 225-248.
Vogt R. 1994. Rhodanthemum laouense
(Compositae, Anthemideae), a new species from Morocco. – Willdenowia 24:
91-96.
Vogt R. 1996. Notes on Asteraceae from N Morocco. –
Lagascalia 18: 301-305.
Vogt R, Oberprieler C. 1995.
Mauranthemum, a new name for Leucoglossum B. H. Wilcox &
al. non S. Imai (Compositae, Anthemideae). – Taxon 44: 377-378.
Vogt R, Oberprieler C. 1996.
Castrilanthemum Vogt & Oberprieler, a new genus of the
Compositae-Anthemideae. – An. Jard. Bot. Madrid 54: 336-346.
Vogt R, Oberprieler C. 2006. The genus
Plagius (Compositae, Anthemideae). – Willdenowia 36 (Spec. issue):
47-68.
Vogt R, Oberprieler C. 2008. Chromosome
numbers of North African phanerogams VIII. More counts in Compositae. –
Willdenowia 38: 497-519.
Voytenko VF. 1989a. Heterocarpy
(heterodiaspory) in angiospermous plants: analysis of the concept,
classification, terminology. – Bot. Žurn. 74: 281-297. [In Russian]
Voytenko VF. 1989b. The typology and
evolution of forms of heterocarpy in the tribe Lactuceae (Asteraceae). – Bot. Žurn. 74:
1241-1257. [In Russian]
Voytenko VF, Oparina SN. 1990. Comparative
analysis of anatomical structure of fruits in heterocarpous representatives of
the tribe Lactuceae (Asteraceae).
– Bot. Žurn. 75: 299-314.
Vuilleumier BS. 1969. The systematics and
evolution of Perezia Sect. Perezia (Compositae). – Contr.
Gray Herb. 199: 1-163.
Wagenitz G. 1955. Pollenmorphologie und
Systematik in der Gattung Centaurea L. s.l. – Flora 142: 213-279.
Wagenitz G. 1963. Die Eingliederung der
”Phaeopappus”-Arten in das System von Centaurea. – Bot.
Jahrb. Syst. 82: 137-215.
Wagenitz G. 1969. Abgrenzung und Gliederung
der Gattung Filago L. s.l. (Compositae-Inuleae). – Willdenowia 5:
395-444.
Wagenitz G. 1970. Die Gattung Filago
L. s.l. (Compositae-Inuleae) in der Ägäis. – Willdenowia 6: 115-138.
Wagenitz G. 1972. Beiträge zur Kenntnis der
Gattung Centaurea L. I. Zur Taxonomie türkischer Arten der Sektionen
Acrolophus und Acrokentron. – Willdenowia 6: 479-508.
Wagenitz G. 1976a. Systematics and phylogeny
of the Compositae (Asteraceae).
– Plant Syst. Evol. 125: 29-46.
Wagenitz G. 1976b. Was ist eine Achäne? Zur
Geschichte eines Karpologischen Begriffs. – Candollea 31: 79-85.
Wagenitz G. 1984. Studies in the flora of
Arabia 7. Centaurea in the Arabian Peninsula. – Notes Roy. Bot.
Gard. Edinb. 41: 457-466.
Wagenitz G. 1986. Centaurea in
South-West Asia: patterns of distribution and diversity. – Proc. Roy. Soc.
Edinb. 89B: 11-21.
Wagenitz G. 1989. Nahe Verwandtschaft
zwischen Arten der Centaurea-Sektionen Acrolophus und
Phalolepis. – Flora 182: 341-351.
Wagenitz G, Bedarff U. 1989. Taxonomic notes
on some species of the genus Cichorium (Compositae-Lactuceae). – In:
Tan K (ed), Plant taxonomy, phytogeography and related subjects. The Davis
& Hedge Festschrift, Edinburgh, pp. 11-21.
Wagenitz G, Gamal-Eldin E. 1983. Die Gattung
Sclerostephane Chiov. (Compositae, Inuleae). – Bot. Jahrb. Syst.
104: 91-113.
Wagenitz G, Gamal-Eldin E. 1985. Zur Kenntnis
der griechischen Centaurea-Arten der Sektion Acrokentron. –
Bot. Jahrb. Syst. 107: 95-127.
Wagenitz G, Hellwig FH. 1996. Evolution of
characters and phylogeny of the Centaureinae. – In: Hind DJN, Beentje HJ
(eds), Compositae: systematics. Proceedings of the International Compositae
Conference, Kew, 1994, I, Royal Botanic Gardens, Kew, pp. 491-510.
Wagenitz G, Hellwig FH. 2000. The genus
Psephellus Cass. (Compositae, Cardueae) revisited with a broadened
concept. – Willdenowia 30: 29-44.
Wagenitz G, Kandemir A. 2008. Two new species
of the genus Psephellus (Compositae, Cardueae) from eastern Turkey.
– Willdenowia 38: 521-526.
Wagenitz G, Dittrich M, Damboldt J. 1982.
Centaurothamnus, eine neue Gattung der Compositae-Cardueae in Arabien.
– Candollea 37: 101-115.
Wagenitz G, Hellwig FH, Parolly G, Martins L.
2006. Two new species of Centaurea (Compositae, Cardueae) from Turkey.
– Willdenowia 36: 423-435.
Wagner H. 1977. Cynareae – chemical review.
– In: Heywood VH, Harborne JB, Turner BL (eds), The biology and chemistry of
the Compositae 2, Academic Press, London, pp. 1017-1038.
Wagner WL, Herbst DR. 1987. A new species of
Remya (Asteraceae-Astereae) on Kaua’i and
a review of the genus. – Syst. Bot. 12: 601-608.
Wagner WL, Lorence DH. 2011. Two new
Marquesan species of the southeastern Polynesian genus Oparanthus (Asteraceae, Coreopsidinae). –
PhytoKeys 4: 139-148.
Wagner WL, Robinson H. 2001.
Lipochaeta and Melanthera (Asteraceae: Heliantheae subtribe
Ecliptinae): establishing their natural limits and a synopsis. – Brittonia
53: 539-561.
Wagner WL, Clark JR, Lorence DH. 2014.
Revision of endemic Marquesas Islands Bidens (Asteraceae, Coreopsideae). –
PhytoKeys 38: 37-67.
Wagstaff SJ, Breitwieser I. 2002.
Phylogenetic relationships of New Zealand Asteraceae inferred from ITS
sequences. – Plant Syst. Evol. 231: 203-224.
Wagstaff SJ, Breitwieser I. 2004. Phylogeny
and classification of Brachyglottis (Senecioneae, Asteraceae): an example of rapid
species radiation in New Zealand. – Syst. Bot. 29: 1003-1010.
Wagstaff SJ, Wege J. 2002. Patterns of
diversification in New Zealand Stylidiaceae. – Amer. J. Bot. 89:
865-874.
Wagstaff SJ, Breitwieser I, Swenson U. 2006.
Origin and relationships of the austral genus Abrotanella (Asteraceae) inferred from DNA
sequences. – Taxon 55: 95-106.
Wahrmund U, Heklau H, Röser M, Kästner A,
Vitek E, Ehrendorfer F, Hagen KB von. 2010. A molecular phylogeny reveals
frequent changes of growth form in Carlina (Asteraceae). – Taxon 59:
367-378.
Waisman CE, Rozenblum E, Hunziker JH. 1986.
Estudios cariológicos en Compositae III. – Darwiniana 27: 179-189.
Wallace RS, Jansen RK. 1990. Systematic
implications of chloroplast DNA variation in the genus Microseris (Asteraceae: Lactuceae). – Syst.
Bot. 15: 606-616.
Walsh NG. 2001. A revision of
Centipeda (Asteraceae).
– Muelleria 15: 33-64.
Wang H, Wortley AH, Blackmore S. 2009. Pollen
morphology of Crepidinae and Lactucinae (Asteraceae: Cichorieae) and its
systematic significance. – Grana 48: 160-178.
Wang L-Y, Pelser PB, Nordenstam B, Liu J-Q.
2009. Strong incongruence between the ITS phylogeny and generic delimitation in
the Nemosenecio-Sinosenecio-Tephroseris assemblage
(Asteraceae: Senecioneae). –
Bot. Stud. 50: 435-442.
Wang Q, Hong D-Y. 2015. Taxonomic revision of
the genus Pseudocodon (Campanulaceae) based on character
analysis and molecular phylogeny. – Phytotaxa 204.1. DOI:
http://dx.doi.org/10.11646/phytotaxa.204.1.4
Wang Q, Zhou S-L, Hong D-Y. 2013. Molecular
phylogeny of the platycodonoid group (Campanulaceae s. str.) with
special reference to the circumscription of Codonopsis. – Taxon 62:
498-504.
Wang Q, Ma X-T, Hong D-Y. 2014. Phylogenetic
analyses reveal three new genera of the Campanulaceae. – J. Syst. Evol.
52: 541-550.
Wang Q, Wang X-Q, Sun H, Yu Y, He X-J, Hong
D-Y. 2014. Evolution of the platycodonoid group with particular references to
biogeography and character evolution. – J. Integr. Plant Biol. 56:
995-1008.
Wang Q, Xu C, Pan K-Y, Hong D-Y. 2017. Which
family? – morphological and phylogenetic analyses of the enigmatic genus
Numaeacampa (Campanulaceae). – Kew. Bull. 72:
72. DOI 10.1007/S12225-017-9701-X
Wang W-M. 2004. On the origin and development
of Artemisia (Asteraceae)
in the geological past. – Bot. J. Linn. Soc. 145: 331-336.
Wang Y-J, Pan J-T, Liu S-W, Liu J-Q. 2005. A
new species of Saussurea (Asteraceae) from Tibet and its
systematic position based on ITS sequence analysis. – Bot. J. Linn. Soc. 147:
349-356.
Wang Y-J, Liu J-Q, Miehe G. 2007.
Phylogenetic origins of the Himalayan endemic Dolomiaea,
Diplazoptilon and Xanthopappus (Asteraceae: Cardueae) based on three
DNA regions. – Ann. Bot. 99: 311-322.
Wang Y-J, Susanna A, Raab-Straube E von,
Milne R, Liu J-Q. 2009. Island-like radiation of Saussurea (Asteraceae: Cardueae) triggered by
uplifts of the Qinghai-Tibetan plateau. – Biol. J. Linn. Soc. 97: 893-903.
Wang Y-J, Raab-Straube E von, Susanna A, Liu
J-Q. 2013. Shangwua (Compositae), a new genus from the Qinghai-Tibetan
Plateau and Himalayas. – Taxon 62: 984-996.
Wang ZT, Xu GJ. 1993. Two new species of
Codonopsis from China. – Acta Phytotaxon. Sin. 31: 184-187.
Warcup HJ, McGee PA. 1983. The mycorrhizal
associations of some Australian Asteraceae. – New Phytol. 95:
667-672.
Ward J, Bayer RJ, Breitwieser I, Smissen R,
Galbany-Casals M, Unwin M. 2009. Gnaphalieae. – In: Funk VA, Susanna A,
Stuessy TF, Bayer RJ (eds), Systematics, evolution, and biogeography of
Compositae, International Association for Plant Taxonomists, Wien, pp.
539-588.
Warwick SI, Gottlieb LD. 1985. Genetic
divergence and geographic speciation in Layia (Compositae). –
Evolution 39: 1236-1241.
Watanabe K, King RM, Tetsukaza Y, Ito M,
Yokoyama J, Suzuki T, Crawford DJ. 1995. Chromosomal cytology and evolution in
Eupatorieae (Asteraceae). – Ann.
Missouri Bot. Gard. 82: 581-592.
Watanabe K, Yahara T, Denda T, Kosuge K.
1999. Chromosomal evolution in the genus Brachyscome (Asteraceae, Astereae): statistical
tests regarding correlation between changes in karyotype and habit using
phylogenetic information. – J. Plant Res. 112: 145-161.
Watanabe K, Short PS, Denda T, Konishi N, Ito
M, Kosuge K. 2000. Chromosome numbers and karyotypes in the Australian
Gnaphalieae and Plucheeae (Asteraceae). – Aust. Syst. Bot. 12:
781-802.
Watanabe K, Kosuge K, Shimamura R, Konishi N,
Taniguchi K. 2006. Molecular systematics of Australian Calotis (Asteraceae: Astereae). – Aust.
Syst. Bot. 19: 155-168.
Watanabe K, Yahara T, Hashimoto G, Nagatani
Y, Soejima A, Kawahara T, Nakazawa M. 2007. Chromosome numbers and karyotypes
in Asteraceae. – Ann. Missouri
Bot. Gard. 94: 643-654.
Watari S. 1939. Anatomical studies on the
leaves of some Saxifragaceous plants, with special reference to the vascular
system. – J. Fac. Sci. Imp. Univ. Tokyo, sect. III, Botany 5: 195-316.
Watson EE. 1929. Contributions to a monograph
of the genus Helianthus. – Pap. Michigan Acad. Sci. 9: 305-475.
Watson LE, Estes JR. 1990. Biosystematic and
phenetic analysis of Marshallia (Asteraceae). – Syst. Bot. 15:
403-414.
Watson LE, Jansen RK, Estes JR. 1991. Tribal
placement of Marshallia (Asteraceae) using chloroplast DNA
restriction site mapping. – Amer. J. Bot. 78: 1028-1035.
Watson LE, Evans TM, Boluarte T. 2000.
Molecular phylogeny of tribe Anthemideae (Asteraceae), based on chloroplast
gene ndhF. – Mol. Phylogen. Evol. 15: 59-69.
Watson LE, Bates PL, Evans TM, Unwin MM,
Estes JR. 2002. Molecular phylogeny of subtribe Artemisiinae (Asteraceae), including
Artemisia and its allied and segregate genera. – BMC Evol. Biol. 2:
17.
Watson TJ. 1977. The taxonomy of
Xylorhiza (Asteraceae-Astereae). – Brittonia
29: 199-216.
Watson TJ. 1978. Chromosome numbers in
Xylorhiza Nuttall (Asteraceae-Astereae). – Madroño
25: 205-210.
Webb CJ. 1994. Pollination,
self-incompatibility, and fruit production in Corokia cotoneaster (Escalloniaceae).
– New Zealand J. Bot. 32: 385-392.
Weber WA, Wittmann RC. 2009.
Delwiensia, a new genus of Asteraceae. – Phytologia 91:
92-94.
Weber XA, Schmidt-Lebuhn AN. 2015. Generic
boundaries of Leucochrysum and Waitzia (Asteraceae: Gnaphalieae). – Aust.
Syst. Bot. 28: 203-218.
Wege JA. 1999. Morphological and anatomical
variation in Stylidium (Stylidiaceae) – a systematic
perspective. – Ph.D. diss., Department of Botany, The University of Western
Australia, Nedlands, Australia.
Wege JA. 2001a. Corolla venation in Stylidiaceae. – J. Roy. Soc.
Western Australia 84: 97-101.
Wege JA. 2001b. Scape anatomy in
Stylidium (Stylidiaceae). – Kew Bull. 56:
955-963.
Wege JA 2004. Chromosome records for five
trigger plants (Stylidium; Stylidiaceae) from northern
Australia. – Austrobaileya 64: 957-959.
Wege JA. 2005. Stylidium validum (Stylidiaceae): a new trigger plant
from southern Western Australia. – J. Roy. Soc. Western Australia 88:
13-16.
Wege JA. 2006a. Stylidium
diplotrichum (Stylidiaceae): a new scale-leaved
trigger plant from south-west Western Australia, with taxonomic and anatomical
notes on allied species. – Nuytsia 16: 183-197.
Wege JA. 2006b. Reinstatement of
Stylidium rigidulum (Stylidiaceae), with notes on the
morphologically allied S. kalbarriense. – Nuytsia 16: 199-206.
Wege JA. 2006c. Taxonomic notes on the locket
trigger plants from Stylidium subgenus Tolypangium section
Repentes. – Nuytsia 16: 207-220.
Wege JA. 2006d. Taxonomic observations on the
Stylidium leptocalyx complex (Stylidiaceae). – Nuytsia 16:
221-231.
Wege JA. 2006e. Taxonomic observations on
Stylidium spathulatum (Stylidiaceae), with the description
of three allied species from section Saxifragoidea. – Nuytsia 16:
233-246.
Wege JA. 2006f. Stylidium
hymenocraspedum (Stylidiaceae) – a new species for
Western Australia, and the lectotypification of S. maitlandianum. –
Nuytsia 16: 247-253.
Wege JA. 2007. New species and new
circumscriptions in Stylidium (Stylidiaceae). – Nuytsia 17:
415-432.
Wege JA. 2008a. Stylidium perplexum
(Stylidiaceae): a remarkable new
triggerplant from south-west Western Australia. – Nuytsia 18: 285-289.
Wege JA. 2008b. Naming Stylidium (Stylidiaceae): an historical
account, with specific reference to S. graminifolium and S.
lineare. – Telopea 12: 321-332.
Wege JA. 2012a. A taxonomic revision of the
Stylidium despectum group (Stylidiaceae) from southern
Australia. – Aust. Syst. Bot. 24: 375-404.
Wege JA. 2012b. Navigating the floral mily
way: the taxonomy of the microgeophytic triggerplants (Stylidium
petiolare and allies: Stylidiaceae). – Aust. Syst. Bot.
25: 138-169.
Wege JA, Coates DJ. 2007. Observations on the
rare triggerplant Stylidium coroniforme (Stylidiaceae) and the description
of two allied taxa of conservation concern. – Nuytsia 17: 433-444.
Wege JA, Keighery GJ, Keighery BJ. 2007. Two
new triggerplants (Stylidium; Stylidiaceae) from the eastern
margin of the Swan Coastal Plain, Western Australia. – Nuytsia 17:
445-452.
Weiler JH Jr. 1962. A biosystematic study of
the genus Downingia. – Ph.D. diss., University of California,
Berkeley, California.
Weiss A. 1890. Untersuchungen über die
Trichome von Corokia budleoides Hort. – Sitzungsber. Kaiserl. Akad.
Wiss., Math.-Naturwiss. Cl. (Wien) 99: 268-282.
Weiss H, Stuessy TF, Grau J, Baeza CM. 2003.
Chromosome reports from South American Hypochaeris (Asteraceae). – Ann. Missouri Bot.
Gard. 90: 56-63.
Weiss-Schneeweiss H, Stuessy TF,
Tremetsberger K, Urtubey E, Valdebenito HA, Beck SG, Baeza CM. 2007. Chromosome
numbers and karyotypes of South American species and populations of
Hypochaeris (Asteraceae).
– Bot. J. Linn. Soc. 153: 49-60.
Weiss-Schneeweiss H, Tremetsberger K,
Schneeweiss GM, Parker JS, Stuessy TF. 2008. Karyotype diversification and
evolution in diploid and polyploid South American Hypochoeris (Asteraceae) inferred from rDNA
localization and genetic fingerprint data. – Ann. Bot. 101: 909-918.
Weiss-Schneeweiss H, Stuessy TF, Villaseñort
JL. 2009. Chromosome numbers, karyotypes, and evolution in Melampodium
(Asteraceae). – Intern. J. Plant
Sci. 170: 1168-1182.
Weitz FM. 1989. A revision of the genus
Corymbium (Asteraceae).
– South Afr. J. Bot. 55: 598-629.
Wells JR. 1965. A taxonomic study of
Polymnia (Compositae). – Brittonia 17: 144-159.
Wendelberger G. 1960. Die Sektion
Heterophyllae der Gattung Artemisia. – Bibl. Bot. 125.
Werker E, Fahn A. 1981. Secretory hairs of
Inula viscosa (L.) Ait. – Development, ultrastructre and secretion.
– Bot. Gaz. 142: 461-476.
Wernham HP. 1912. Floral evolution with
particular reference to the sympetalous dicotyledons VIII. Inferae, part ii,
Campanulatae. – New Phytol. 11: 290-305.
Wetter MA. 1983. Micromorphological
characters and generic delimitation of some New World Senecioneae (Asteraceae). – Brittonia 35:
1-22.
Whitaker TW, Steyermark JA. 1935. Cytological
aspects of Grindelia species. – Bull. Torrey Bot. Club 62: 69-73.
Whitehouse C. 1996. Menyanthaceae. – In: Polhill RM
(ed), Flora of tropical East Africa, A. A. Balkema, Rotterdam, The Netherlands,
pp. 1-7.
Whitkus R. 1998. Genetics of adaptive
radiation in Hawaiian and Cook Islands species of Tetramolopium (Asteraceae) II. Genetic linkage map
and its implicaions for interspecific breeding barriers. – Genetics 150:
1209-1216.
Whitton J, Wallace RS, Jansen RK. 1995.
Phylogenetic relationships and patterns of character change in the tribe
Lactuceae (Asteraceae) based on
chloroplast DNA restriction site variation. – Can. J. Bot. 73: 1058-1073.
Widder FJ. 1923. Die Arten der Gattung
Xanthium. Beiträge zu einer Monographie. – Feddes Repert. Beih.
20.
Widder FJ. 1931. Beiträge zur Kenntnis der
Gattung Leontodon II. Die “nickenden Knospen” einiger
Leontodon-Arten in ihrer Bedeutung für das System der Gattung. –
Österr. Bot. Zeitschr. 80: 136-148.
Widder FJ. 1967. Ostafrikanische
Xanthium-Arten. – Phyton (Horn) 12: 182-190.
Widder FJ. 1975. Die Gliderung der Gattung
Leontodon. – Phyton (Horn) 17: 23-29.
Wiklund A. 1983. Ighermia, a new
genus of the Asteraceae-Inuleae.
– Nord. J. Bot. 3: 443-446.
Wiklund A. 1985. The genus
Asteriscus (Asteraceae-Inuleae). – Nord. J.
Bot. 5: 299-314.
Wiklund A. 1987a. The genus Nauplius
(Asteracee-Inuleae). – Nord. J. Bot. 7: 1-23.
Wiklund A. 1987b. The genus
Rhanteriopsis (Asteraceae-Inuleae). – Bot. J.
Linn. Soc. 95: 27-42.
Wiklund A. 1992. The genus Cynara L.
(Asteraceae-Cardueae). – Bot. J.
Linn. Soc. 109: 75-123.
Wiklund A. Moth. 2003. Arcyna, a new
genus segregated from Cynara (Compositae). – Willdenowia 33:
63-68.
Wild H. 1964. A revision of the genus
Anisopappus Hook. & Arn. (Compositae). – Kirkia 4: 45-73.
Wild H. 1969. The genus Nidorella
Cass. – Bol. Soc. Brot. 43: 209-243.
Wild H. 1973. A new genus of Compositae
(Astereae) from the flora Zambesiaca area. – Garcia de Orta 1: 67-68.
Wild H. 1974. New and interesting Compositae
from South-Central Africa 2. – Kirkia 9: 293-300.
Wild H. 1980. The Compositae of the Flora
Zambesiaca area 12 – Inuleae (continued). – Kirkia 12: 23-113.
Willis AJ, Ash JE. 1990. The breeding system
of Stylidium graminifolium and S. productum (Stylidiaceae). – Aust. J. Bot.
38: 217-227.
Willis JH. 1956. Sundry notes on Australian
triggerplants (including a new name for a tropical triggerplant). – Victorian
Natur. 73: 43-44.
Willis JH. 1967. Notes on two species of
western Australian Compositae. – West. Aust. Bot. 10: 157-158.
Wilson FD. 1982. A cytological basis for the
separation of Geropogon from Tragopogon (Compositae,
Lactuceae). – Brittonia 34: 290-293.
Wilson PG. 1989. A revision of the genus
Hyalosperma (Asteraceae:
Inuleae: Gnaphaliinae). – Nuytsia 7: 75-101.
Wilson PG. 1992a. The Lawrencella
complex (Asteraceae: Gnaphalieae:
Angianthinae) of Australia. – Nuytsia 8: 361-377.
Wilson PG. 1992b. The classification of
Australian species currently included in Helipterum and related genera
(Asteraceae: Gnaphalieae): part 1.
– Nuytsia 8: 379-438.
Wilson PG. 1992c. The classification of
Australian species currently included in Helipterum (Asteraceae: gnaphalieae): part 2.
Leucochrysum. – Nuytsia 8: 439-446.
Wilson PG. 1992d. The classification of some
Australian species currently included in Helipterum and
Helichrysum (Asteraceae:
Gnaphalieae): part 3. Anemocarpa and Argentipallium, two new
genera from Australia. – Nuytsia 8: 447-460.
Wilson PG. 2001. Leiocarpa, a new
Australian genus of the Asteraceae
tribe Gnaphalieae. – Nuytsia 13: 595-605.
Wimmer FE. 1948. Vorarbeiten zur Monographie
der Campanulaceae-Lobelioideae
II. Trib. Lobelieae. – Ann. Naturhist. Mus. Wien 56: 317-374.
Wimmer FE. 1952. Herbarium africanum: novae
species e generibus Lobeliae, Monopsis, Cyphiae. –
Kew Bull. 1952: 137-147.
Wimmer FE. 1953. 186e famille.
Lobéliacées. – In: Humbert H (ed), Flore de Madagascar et des Comores,
Muséum National Histoire Naturelle, Paris.
Winkler C. 1892. Synopsis specierum generis
Cousiniae. – Trudy Imp. S.-Peterburgsk. Bot. Sada 12: 181-286.
Winkler C. 1897. Mantissa synopsis specierum
generis Cousiniae. – Trudy Imp. S.-Peterburgsk. Bot. Sada 14:
187-243.
Winkworth RC, Lundberg J, Donoghue MJ. 2008.
Toward a resolution of campanulid phylogeny, with special reference to the
placement of Dipsacales.
– Taxon 57: 53-65.
Witter MS. 1986. Genetic differentiation in
the Hawaiian silversword alliance (Compositae: Madiinae). – Ph.D. diss.,
University of Hawaii, Honolulu, Hawaii.
Witter MS. 1990. Evolution in the Madiinae:
evidence from enzyme electrophoresis. – Ann. Missouri Bot. Gard. 77:
110-117.
Witter MS, Carr GD. 1988. Adaptive radiation
and genetic differentiation in the Hawaiian silversword alliance (Compositae:
Madiinae). – Evolution 42: 1278-1287.
Wodehouse RP. 1928a. Pollen grains in the
identification and classification of plants I. The Ambrosiaceae. – Bull.
Torrey Bot. Club 55: 181-198.
Wodehouse RP. 1928b. Pollen grains in the
identification and classification of plants II. Barnadesia. – Bull.
Torrey Bot. Club 55: 449-462.
Wodehouse RP. 1929a. Pollen grains in the
identification and classification of plants III. The Nassauviinae. – Bull.
Torrey Bot. Club 56: 123-137.
Wodehouse RP. 1929b. Pollen grains in the
identification and classification of plants IV. The Mutisieae. – Amer. J.
Bot. 16: 297-313.
Wolf SJ. 1987. Cytotaxonomic studies in the
genus Arnica (Compositae: Senecioneae). – Rhodora 89: 391-400.
Wood AR, Nordenstam B. 2004. An interesting
new species of Osteospermum (Asteraceae-Calenduleae) from the
Western Cape Province, South Africa, providing a link to the genus
Chrysanthemoides. – South Afr. J. Bot. 69: 572-578.
Wood CE Jr. 1961. A study of hybridization in
Downingia (Campanulaceae). – J. Arnold
Arbor. 42: 219-262.
Wortley AH, Funk VA, Robinson H, Skvarla JJ,
Blackmore S. 2007. A search for pollen morphological synapomorphies to classify
rogue genera in Compositae (Asteraceae). – Rev. Palaeobot.
Palynol. 146: 169-181.
Wortley AH, Funk VA, Skvarla JJ. 2008. Pollen
and the evolution of Arctotideae (Compositae). – Bot. Rev. 74: 438-466.
Wortley AH, Blackmore S, Chissoe WF, Skvarla
JJ. 2012. Recent advances in Compositae (Asteraceae) palynology, with emphasis
on previously unstudied and unplaced taxa. – Grana 51: 158-179.
Wulff AF, Hunziker JH, Escobar A. 1996.
Estudios cariológicos en Compositae VII. – Darwiniana 34: 213-231.
Wussow JR, Urbatsch LE. 1979. A systematic
study of the genus Tetrachyron (Asteraceae: Heliantheae). – Syst.
Bot. 4: 297-318.
Wussow JR, Urbatsch LE, Sullivan GA. 1985.
Calea (Asteraceae) in
Mexico, Central America, and Jamaica. – Syst. Bot. 10: 241-267.
Wysk R, Nordenstam B, Kadereit JW, Westberg
E. 2009. The identity and geographical distgribution of Jacobaea
vulgaris subsp. gotlandica, supposedly endemic to Gotland and
Öland (Sweden) – the importance of multiple intraspecific samples. – Taxon
58: 1133-1140.
Xiaoping Z, Bremer K. 1993. A cladistic
analysis of the tribe Astereae (Asteraceae) with notes on their
evolution and subtribal classification. – Plant Syst. Evol. 184: 259-283.
Xu X, Zheng W, Funk VA, Li K, Zhang J, Wen J.
2018. Home at last III: transferring Uechtritzia and Asian
Gerbera species into Oreoseris (Compositae Mutisieae). –
PhytoKeys 96: 1-19.
Yahara T, Kawahara T, Crawford DJ, Ito M,
Watanabe K. 1989. Extensive gene duplication in diploid Eupatorium (Asteraceae). – Amer. J. Bot. 76:
1247-1253.
Yavin Z. 1970. A biosystematical study of
Anthemis section Maruta (Compositae). – Israel J. Bot. 19:
137-154.
Yavin Z. 1972. New taxa of Anthemis
from the Mediterranean and SW Asia. – Israel J. Bot. 21: 168-178.
Yeo PF. 1993a. Platycodoneae: a new tribe in
Campanulaceae. – Taxon 42:
109.
Yeo PF. 1993b. Secondary pollen presentation.
Form, function and evolution. – Plant Syst. Evol. [Suppl.] 6: 111-129.
Yildiz B, Dirmenci T. 2009. A new species of
Cirsium section Epitrachys (Asteraceae: Cardueae) from Turkey.
– Bot. J. Linn. Soc. 158: 669-673.
Zanotti CA, Pozner RE. 2007. Valor
diagnóstico de la estructura del fruto de Boopis y
Nastanthus (Calyceraceae). – Bol. Soc.
Argent. Bot. 42(Suppl.): 142.
Zardini EM. 1974. Sobre la presencia del
género Gerbera en America. – Bol. Soc. Argent. Bot. 16: 103-108.
Zardini EM. 1975. Revisión del género
Trichocline (Compositae). – Darwiniana 19: 618-733.
Zardini EM. 1976. Nota acerca de dos nombres
en el género Trichocline Cass. (Compositae). – Darwiniana 20:
304.
Zardini EM. 1980. Lulia – un nuevo
género de Compuestas. – Bol. Soc. Argent. Bot. 19: 255-258.
Zardini EM. 1981. Contribuciones para una
monografía del género Conyza Less. II. Rehabilitación del género
Laennecia Cass. – Darwiniana 23: 159-169.
Zardini EM. 1985. Revision del género
Noticastrum (Compositae-Astereae). – Rev. Mus. La Plata, Secc. Bot.
13: 313-424.
Zareh MM. 2005. Systematic and anatomical
studies of Inuleae and Plucheeae in Egypt. – Feddes Repert. 116: 43-53.
Zareh MM. 2009. Systematic revision of
Compositae in Egypt 10. Tribe Anthemideae. – Feddes Repert. 120: 15-26.
Zavada MS, Lowrey TK. 2010. Comparative
pollen morphoogy of Brachylaena, Tarchonanthus and two
species of Tubulifloridites (Asteraceae) from the Eocene, Knysna
Lignite of South Africa. – Rev. Palaeobot. Palynol. 162: 183-192.
Zavada MS, Villiers SE de. 2000. Pollen of
the Asteraceae from the
Paleocene-Eocene of South Africa. – Grana 39: 39-45.
Zavala-Gallo L, Denham S, Pozner R. 2010.
Revision of the genus Nastanthus (Calyceraceae). – Gayana Bot. 67:
158-175.
Zavala-Gallo L, Denham S, Pozner R. 2011. Two
new species of Boopis (Calyceraceae) from Argentina. –
Brittonia 63: 113-117.
Zdero C, Bohlmann F. 1987. Sesquiterpene
lactones from the genus Brachylaena. – Phytochemistry 26:
2597-2601.
Zdero C, Bohlmann F. 1988. Macrolide
diterpenes and other ent-labdanes from Corymbium villosum. –
Phytochemistry 27: 227-231.
Zdero C, Bohlmann F. 1990. Systematics and
evolution within the Compositae, seen with the eyes of a chemist. – Plant
Syst. Evol. 171: 1-14.
Zdero C, Bohlmann F, King RM, Robinson H.
1986. Further 5-methyl coumarins and other constituents from the subtribe
Mutisiinae. – Phytochemistry 25: 509-516.
Zdero C, Bohlmann F, Mungai GM. 1990. A
glaucolide-type sesquiterpene lactone from Vernonia galamensis ssp.
nairobensis. – Phytochemistry 29: 3668-3669.
Zdero C, Bohlmann F, Anderberg AA, King RM.
1991. Eremophilane derivatives and other constituents from Haeckeria
species and further Australian Inuleae. – Phytochemistry 30: 2643-2650.
Zdero C, Bohlmann F, Anderberg AA. 1991a.
Kaurane succinates and prenylated aromatics from Odixia angusta and
Ozothamnus obcordatus. – Phytochemistry 30: 2703-2706.
Zdero C, Bohlmann F, Anderberg AA. 1991b.
Leysseral derivatives from Anisothrix integra and Phagnalon
purpurascens. – Phytochemistry 30: 3009-3011.
Zeman M. 1907. Studien zu einer Monographie
der Gattung Argophyllum Forst. – Ann. K. K. Naturhist. Hofmus. 22:
270-292.
Zhang J-W, Sun H, Nie Z-L. 2007. Karyological
studies on the Sino-Himalayan endemic Soroseris and two related genera
of tribe Lactuceae (Asteraceae).
– Bot. J. Linn. Soc. 154: 79-87.
Zhang J-W, Nie Z-L, Wen J, Sun H. 2011.
Molecular phylogeny and biogeography of three closely related genera,
Soroseris, Stebbinsia, and Syncalathium (Asteraceae, Cichorieae), endemic to
the Tibetan Plateau, SW China. – Taxon 60: 15-26.
Zhang J-W, Boufford DE, Sun H. 2011.
Parasyncalathium J. W. Zhang, Boufford & H. Sun (Asteraceae, Cichorieae): a new genus
endemic to the Himalaya-Hengduan Mountans. – Taxon 60: 1678-1684.
Zhang J-W, Boufford DE, Sun H. 2013.
Systematic significance of achene morphology in Soroseris,
Syncalathium and Parasyncalathium (Asteraceae: Cichorieae). – Bot. J.
Linn. Soc. 173: 476-486.
Zhang X-P, Bremer K. 1993. A cladistic
analysis of the tribe Astereae (Asteraceae) with notes on their
evolution and subtribal classification. – Plant Syst. Evol. 184: 259-283.
Zhao H-B, Chen F-D, Chen S-M, Wu G-S, Guo
W-M. 2010. Molecular phylogeny of Chrysanthemum, Ajania and
its allies (Anthemideae, Asteraceae) as inferred from nuclear
ribosomal ITS and chloroplast trnL-F IGS sequences. – Plant Syst.
Evol. 284: 153-169.
Zhao Z, Skvarla JJ, Jansen RK, DeVore ML.
2000. Phylogenetic implications of pollen morphology and ultrastructure in the
Barnadesioideae (Asteraceae). –
Lundellia 3: 26-40.
Zhao Z, Skvarla JJ, Jansen RK. 2006.
Mutisieae (Asteraceae) pollen
ultrastructure atlas. – Lundellia 9: 51-76.
Zhou Z, Wen J, Li G, Sun H. 2012.
Phylogenetic assessment and biogeographic analyses of tribe Peracarpeae (Campanulaceae). – Plant Syst.
Evol. 298: 323-336.
Zhou Z, Hong D, Niu Y, Li G, Nie ZL, Wen
J,Sun H. 2013. Phylogenetic and biogeographic analyses of the Sino-Himalayan
endemic genus Cyananthus (Campanulaceae) and implications
for the evolution of its sexual system. – Mol. Phylogen. Evol. 68:
482-497.
Zhu Y, Zhao Y, Meng X-H, Wu W-S. 2009.
Chemical constituents from Sinacalia tangutica. – Biochem. Syst.
Ecol. 37: 59-62.
Zidorn C, Stuppner H. 2001. Evaluation of
chemosystematic characters in the genus Leontodon (Asteraceae). – Taxon 50:
115-133.
Zidorn C, Gottschlich G, Stuppner H. 2002.
Chemosystematic investigations on phenolics from flowerheads of Central
European taxa of Hieracium sensu lato (Asteraceae). – Plant Syst. Evol.
231: 39-58.
Zohary M. 1950. Evolutionary trends in the
fruiting head of Compositae. – Evolution 4: 103-109.










