APIIDAE
M. J. Donoghue et P. D. Cantino
Donoghue et Cantino in Taxon 56: E31. Aug 2007
[Escalloniaceae+[Bruniales+Dipsapiidae]]
ESCALLONIACEAE R. Br. ex
Dumort.
Dumortier, Anal. Fam. Plant.: 35, 37. 1829, nom.
cons.
Escalloniales R.
Brown in C. F. P. von Martius, Consp. Regn. Veg.: 47. Sep-Oct 1835
[’Escallonieae’]; Polyosmaceae Blume,
Mus. Bot. 1: 258. 1851; Eremosynaceae Dandy in J.
Hutchinson, Fam. Fl. Pl., ed. 2: 460. 4 Jun 1959;
Tribelaceae (Engl.) Airy Shaw in Kew Bull. 18: 269. 8
Dec 1965; Anopteraceae Doweld, Tent. Syst. Plant.
Vasc.: li. 23 Dec 2001; Escallonianae Doweld, Tent.
Syst. Plant. Vasc.: li. 23 Dec 2001; Tribelales
Doweld, Tent. Syst. Plant. Vasc.: li. 23 Dec 2001;
Escalloniineae Shipunov in A. Shipunov et J. L.
Reveal in Phytotaxa 16: 63. 4 Feb 2011
Genera/species 7/105–110
Distribution South America,
with their largest diversity in the Andes, the Mascarene Islands, southwestern
West Australia, southeastern Australia, Tasmania, southern Chile to Tierra del
Fuego, eastern Himalayas and southern China to Southeast Asia, Malesia to New
Guinea, tropical Australia, New Caledonia.
Fossils Fossil leaves, flowers
and fruits of Escallonia have been reported at least from Eocene and
Early Miocene layers in Chile.
Habit Usually bisexual (rarely
unisexual), usually evergreen trees and shrubs (Valdivia gayana is a
small shrub; Tribeles australis is a low procumbent and creeping
shrub; Eremosyne pectinata is an annual herb).
Vegetative anatomy Phellogen
usually superficial (in Escallonia ab initio deeply seated). Narrow
primary medullary rays alternating with wide rays. Endodermis absent. Vascular
cylinder complete in Forgesia. Vessel elements usually with
scalariform (in Eremosyne and some species of Escallonia
simple) perforation plates (in Polyosma scalariform with very numerous
transverse ribs); lateral pits alternate, scalariform or opposite, bordered
pits. Imperforate tracheary xylem elements tracheids (at least in
Forgesia and Polyosma also fibre tracheids) with bordered
pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or
multiseriate, homocellular or heterocellular (sometimes storied). Axial
parenchyma usually apotracheal diffuse or diffuse-in-aggregates (at least in
Forgesia and Polyosma also paratracheal scanty). Sieve tube
plastids S type. Nodes in Eremosyne, Escallonia,
Polyosma, and Tribeles 1:1, unilacunar with one leaf trace,
in Anopterus, Forgesia and Valdivia 3:3, trilacunar
with three traces. Pericycle in Polyosma with sclereids; pericyclic
envelope in Forgesia with sclereids. Secretory cavities absent.
Heartwood in some species with gum-like substances. Styloids and/or elongate
calciumoxalate crystals present in some species of Escallonia.
Druses present in Polyosma andTribeles, and in
cortex of Forgesia.
Trichomes Hairs usually
unicellular (in Anopterus and Valdivia also multicellular,
simple, uniseriate; in Polyosma crescent-shaped with base perforated
and sunken into epidermis), thick-walled (sometimes furcate); glandular hairs
with uniseriate stalk and globular head (sometimes peltate-lepidote; glandular
hairs in Escallonia also multiseriate with multicellular head); cells
in heads of glandular hairs with vertically radiating walls.
Leaves Usually alternate
(spiral; in Polyosma opposite), simple, usually entire (in
Eremosyne usually pinnately to palmately lobed), often coriaceous,
usually with supervolute (sometimes conduplicate) ptyxis. Stipules usually
absent (Escallonia sometimes with prickly stipules); leaf sheath
absent. Petiole vascular bundle transection arcuate. Venation usually pinnate
(in Eremosyne palmate, acrodromous), usually semicraspedodromous (in
Polyosma often brochidodromous, in Anopterus and
Forgesia intermediate between eucamptodromous and
semicraspedodromous). Stomata usually anomocytic (sometimes paracytic).
Cuticular wax crystalloids? Druses present in Polyosma. Leaf margin
entire, crenate, serrate or biserrate, often with wide glandular teeth and two
accessory veins; apical cells of glandular hairs radially arranged.
Inflorescence Terminal or
axillary, usually racemes (in Eremosyne terminal cyme consisting of
dichasia; flowers in Tribeles solitary terminal). Floral prophylls
(bracteoles) absent in Eremosyne.
Flowers Actinomorphic. Usually
epigyny (sometimes hypogyny; in Eremosyne and Tribeles
partial epigyny). Sepals usually five (in Anopterus six to nine; in
Valdivia five to seven; in Polyosma four), with valvate or
imbricate aestivation (in Eremosyne minute, with valvate aestivation;
in Tribeles with imbricate aestivation), usually more or less connate
and persistent. Petals usually five (in Valdivia five to seven; in
Anopterus six to nine; in Polyosma four), usually with
imbricate aestivation (in Forgesia and Valdivia valvate; in
Tribeles shortly clawed, with contorted aestivation; in
Polyosma four, with valvate aestivation), free, deciduous.
Nectariferous disc intrastaminal (absent in Eremosyne and
Tribeles).
Androecium Stamens usually
five (in Valdivia five to seven; in Anopterus six to nine; in
Polyosma four), haplostemonous, antesepalous, alternipetalous.
Filaments in Eremosyne and Tribeles subulate, free from each
other and from petals. Anthers usually dorsifixed (rarely basifixed), often
versatile, disporangiate, usually introrse (in Eremosyne introrse to
latrorse, versatile; in Tribeles extrorse), longicidal (dehiscing by
longitudinal slits). Tapetum secretory, with binucleate cells
(Escallonia). Placentoid present. Staminodia absent.
Pollen grains
Microsporogenesis simultaneous. Pollen grains usually tricolporate (in
Polyosma triporate; in Anopterus sometimes tetracolporate),
shed as monads, bicellular at dispersal. Exine tectate or semitectate, with
columellate infratectum, usually perforate to reticulate or finely
reticulate.
Gynoecium Pistil composed of
usually two (sometimes four; in Tribeles three) connate carpels;
transverse or median carpel adaxial (ovary median in Escallonia).
Ovary usually inferior (sometimes semi-inferior or superior), usually partially
bilocular with incomplete septa in lower part (in Eremosyne bilocular;
in Tribeles trilocular; in Polyosma usually unilocular).
Style usually single and simple, sometimes bifid or trifid (stylodia in
Eremosyne two, free or connate at base; in Anopterus and
Forgesia more or less free), often long. Stigma capitate, punctate or
clavate to bilobate to quinquelobate, non-papillate, Wet type. Pistillodium
absent.
Ovules Placentation usually
parietal (sometimes more or less basal; in Polyosma strongly
intrusively parietal; in Eremosyne and Tribeles axial).
Ovules usually numerous (in Eremosyne one, basal, ascending) per
carpel, usually anatropous (in Eremosyne campylotropous), anatropous,
usually unitegmic (in Tribeles bitegmic), usually tenuinucellar (in
Polyosma often almost crassinucellar). Micropyle in Tribeles
?-stomal. Integument five to ten (in Polyosma up to ten) cell layers
thick. Outer integument in Tribeles ? cell layers thick. Inner
integument in Tribeles ? cell layers thick. Hypostase absent. Parietal
tissue approx. one cell layer thick (Polyosma). Megagametophyte
monosporous, Polygonum type. Endosperm development nuclear (or
cellular?). Endosperm haustorium micropylar. Embryogenesis?
Fruit Usually a septicidal (in
Eremosyne and Tribeles loculicidal) capsule, often dehiscing
laterally (fruit in Valdivia dry, indehiscent; in Polyosma a
one-seeded drupe).
Seeds Aril present in
Eremosyne. Testa in Anopterus winged. Exotestal cells usually
with inner walls thickened (in, e.g., Anopterus) and lignified, often
elongate (in, e.g., Anopterus; in Tribeles palisade).
Perisperm not developed. Endosperm copious, fleshy, usually oily (in
Polyosma thick-walled, starchy). Embryo usually small, straight,
usually well differentiated (in Polyosma undifferentiated),
chlorophyll absent (Tribeles). Cotyledons two. Germination?
Cytology n = 9
(Eremosyne), 12 (Escallonia)
DNA Mitochondrial intron
coxII.i3 lost (Escallonia). I copy of nuclear gene
RPB2 present (Escallonia).
Phytochemistry Flavonols
(kaempferol, quercetin), cyanidin, delphinidin, Route I secoiridoids (e.g.
daphylloside), Route II decarboxylated iridoids, diterpene alkaloids
(anopterine etc. in Anopterus), triterpenes and saponins present
(ursolic acid, a pentacyclic triterene, present in Escallonia).
Ellagic acid and cyanogenic compounds not found. Aluminium accumulated
in Polyosma.
Use Ornamental plants
(Escallonia), timber (Polyosma), dyeing substances.
Systematics Eremosyne
(1; E. pectinata; southwesternmost Western Australia),
Anopterus (2; A. glandulosus: western and southern Tasmania;
A. macleayanus: southeasternmost Queensland, northeastern New South
Wales), Polyosma (c 60; eastern Himalayas and southern China to
eastern Queensland, eastern New South Wales and New Caledonia),
Tribeles (1; T. australis; temperate Chile and Argentina), Escallonia
(c 40; southern Central America, South America, with their highest diversity in
the Andes), Forgesia (1; F. racemosa; Réunion),
Valdivia (1; V. gayana; near Valdivia in central Chile). –
Southern Central America to southern Chile, Réunion, southeastern and
southwestern Australia, Tasmania. Usually trees or shrubs (rarely annual
herbs). Phellogen in Escallonia often deeply seated. Nodes usually 3:3
(sometimes 1:1 or 5:5). Cells of glandular heads with radially arranged walls.
Leaves spiral, with usually supervolute (sometimes conduplicate) ptyxis.
Petiole vascular bundle transection arcuate. Stomata anomocytic. Leaf margin
usually serrate, with glandular teeth and two accessory veins (leaf apex in
Tribeles with three small teeth). Petals in Escallonia
connate. Anthers often longer than connective. Placentoid sometimes present.
Endoaperture in Eremosyne
complex. Tectum in Eremosyne
and Tribeles incomplete, in Tribeles rugulate-reticulate with
disrupted muri. Stigma in Tribeles subclavate to somewhat trilobate.
Ovules usually unitegmic (in Tribeles bitegmic). Integument five to
eight cell layers thick. Micropyle often elongated. Seeds in Tribeles
long attached to central columella of open ripe coriaceous capsule. Mesotesta
and/or endotesta usually persistent. Embryo sometimes elongated. Both copies of
nuclear gene rpb2 present in Escallonia.
Flavonols, Route I secoiridoids and Route II decarboxylated iridoids
present.
Escalloniaceae are probably
sister-group to the clade [Bruniales+[Araliales+[Paracryphiales+Dipsacales]]].
The results from the different molecular
analyses of Escalloniaceae are
contradictory. Polyosma was recovered as sister-group to
Quintinia (Paracryphiaceae),
with high bootstrap support, when using mitochondrial DNA data in the 17-gene
analyses by Soltis & al. (2011). On the other hand, when mtDNA data were
excluded, Polyosma was placed in Escalloniaceae, also with
high bootstrap support. Horizontal transfer of mitochondrial genes may explain
these results, according to Soltis & al. (2011). Moreover,
Polyosma was sister to the remaining Escalloniaceae in the
analysis by Lundberg (2001). Finally, it was closely allied to
Anopterus and Tribeles in the investigation by Sede & al.
(2013), whereas Eremosyne
was sister to the remaining Escalloniaceae. –
Polyosma consists of trees. Phellogen subepidermal. Pericycle with
sclereids. Nodes 1:1, unilacunar with a single leaf trace. Hairs unicellular.
Leaves opposite. Leaf margin often serrate. Stipules absent. Inflorescence
axillary raceme. Epigyny. Flowers with long buds. Sepals four, connate. Petals
four, with valvate aestivation, free (primarily or secondarily?), relatively
narrow. Pollen grains triporate. Pistil composed of two connate carpels. Ovary
inferior. Stigma capitate, bilobate. Placentation intrusively parietal. Ovules
numerous per carpel. Integument up to ten cell layers thick. Parietal tissue
approx. one cell layer thick? Megasporangial base massive. Megasporocytes
sometimes several. Fruit a single-seeded drupe. Testa up to ten cell layers
thick. Endosperm thick-walled, rich in starch. Embryo undifferentiated. n = ?
Iridoids present. Aluminium accumulated in at least some species.
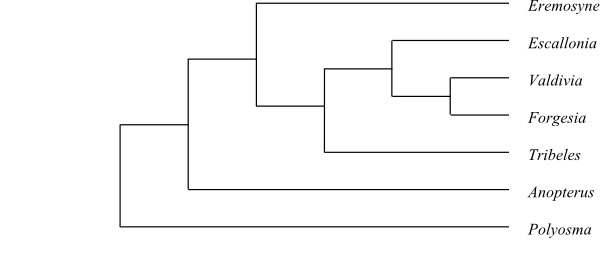 |
Phylogeny of Escalloniaceae
based on DNA sequence data (Lundberg 2001).
|
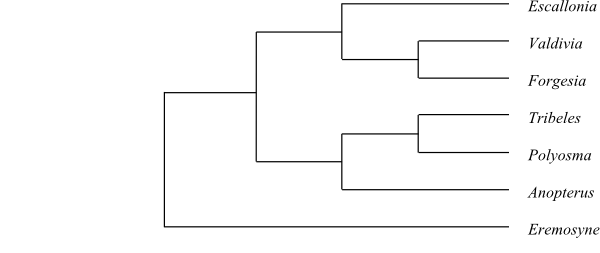 |
Phylogeny (majority-rule consensus tree) of Escalloniaceae
based on DNA sequence data (Sede & al. 2013).
|
Literature
Al-Shammary KI. 1991. Systematic studies of
the Saxifragaceae,
chiefly from the Southern Hemisphere. – Ph.D. diss., University of Leicester,
Leicester, United Kingdom.
Al-Shammary KI, Gornall RJ. 1994. Trichome
anatomy of the Saxifragaceae
s.l. from the Southern Hemisphere. – Bot. J. Linn. Soc. 114: 99-131.
Aplin THE, Cannon JR. 1971. Distribution of
alkaloids in some Western Australian plants. – Econ. Bot. 25: 366-380.
Bensel CR, Palser BF. 1975. Floral anatomy in
the Saxifragaceae
sensu lato III. Kirengeshomoideae, Hydrangeoideae and Escallonioideae. –
Amer. J. Bot. 62: 676-687.
Boer E, Sosef MSM. 1998. Polyosma
Blume. – In: Sosef MSM, Hong L-T, Prawirohatmodjo S (eds), Plant resources of
South-East Asia 5(3). Timber trees: lesser-known timber, Backhuys Publ.,
Leiden, pp. 464-465.
Dandy JE. 1927. The genera of Saxifragaceae.
– Kew Bull. 1927: 107-118.
Dawson MI. 1995. Contributions to a
chromosome atlas of the New Zealand flora 33. Miscellaneous species. – New
Zealand J. Bot. 33: 477-487.
Engler A. 1891. Saxifragaceae.
– In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2a), W.
Engelmann, Leipzig, pp. 41-93.
Engler A. 1930. Saxifragaceae.
– In: Engler A, Harms H (eds), Die natürlichen Pflanzenfamilien, 2. Aufl.,
Bd. 18a, W. Engelmann, Leipzig, pp. 74-226.
Gelius L. 1967. Studien zur
Entwicklungsgeschichte an Blüten der Saxifragales sensu
lato mit besonderer Berücksichtigung des Androeceums. – Bot. Jahrb. Syst.
87: 253-303.
Goodson JA. 1938. The occurrence of ursolic
acid in Escallonia tortuosa. Conversion of ursolic acid into
alpha-amyrin. – J. Chem. Soc. 1938: 999-1001.
Gornall RJ, Al-Shammary KIA, Gregory M. 1998.
Escalloniaceae. – In:
Cutler DF, Gregory M (eds), Anatomy of the dicotyledons, 2nd ed.,
IV. Saxifragales,
Clarendon Press, Oxford, United Kingdom, pp. 41-86.
Gunckel H. 1931. Contribución al
conocimiento de la flora valdiviana (quinta comunicación): Valdivia
gayana Rémy. – Rev. Univ. (Santiago) 16: 510-517.
Hamel JL. 1953. Contribution à l’étude
cytotaxinomique des Saxifragacées. – Rev. Cytol. Biol. Vég. 14: 113-313.
Hibsch-Jetter C, Soltis DE, Macfarlane TD.
1997. Phylogenetic analysis of Eremosyne pectinata (Saxifragaceae
s.l.) based on rbcL sequence data. – Plant Syst. Evol. 204:
225-232.
Hideux M, Ferguson IK. 1976. The
stereostructure of the exine and its evolutionary significance in Saxifragaceae
sensu lato. – In: Ferguson IK, Muller J (eds), The evolutionary significance
of the exine, Linn. Soc. Symposium, No. 1, Academic Press, London and New York,
pp. 327-377.
Hils MH. 1985. Comparative anatomy and
systematics of twelve woody Australasian genera of the Saxifragaceae.
– Ph.D. diss., University of Florida, Gainesville, Florida.
Holle G. 1893. Beiträge zur Anatomie der
Saxifragaceen und deren systematische Verwerthung. – Bot. Centralbl. 53: 1-9,
33-41, 65-70, 97-102, 129-136, 161-169, 209-222.
Jay M. 1969. Contribution biochimique à la
connaissance taxonomique et phylogénetique des Saxifragacées et familles
affinés. – Ph.D. diss., l’Université de Lyon, France.
Kadereit JW, Bittrich V (eds). 2016. The
families and genera of vascular plants XIV. Flowering plants – eudicots –
Aquifoliales, Boraginales, Bruniales, Dipsacales, Escalloniales, Garryales, Paracryphiales,
Solanales (except Convolvulaceae), Icacinaceae, Metteniusaceae,
Vahliaceae. – Springer,
412 pp.
Kamelina OP. 1984. On the embryology of the
genus Escallonia. – Bot. Žurn. 69: 1304-1316. [In Russian]
Kamelina OP. 1988. Sporo-, gametogenesis, and
fertilization of Escallonia and Brexia with comments on their
taxonomy. – In: Cresti M, Gori P, Pacini E (ed), Sexual reproduction in
higher plants, Springer, Berlin, pp. 431-435.
Klopfer K. 1973. Florale Morphogenese und
Taxonomie der Saxifragaceae
sensu lato. – Feddes Rep. 84: 475-516.
Krach JE. 1976. Samenanatomie der Rosifloren
I. Die Samen der Saxifragaceae.
– Bot. Jahrb. Syst. 97: 1-60.
Krach JE. 1977. Seed characters in and
affinities among the Saxifragaceae.
– Plant Syst. Evol. [Suppl.] 1: 141-153.
Lundberg J. 2001. Escalloniaceae. – In: Lundberg
J, Phylogenetic studies in the euasterids II, with particular reference to
Asterales and Escalloniaceae,
Ph.D. diss., Acta Universitatis Upsaliensis, Uppsala, Sweden.
Mai DH. 1985. Beiträge zur Geschichte
einiger holziger Saxifragales-Gattungen.
– Gleditschia 13: 75-88.
Mauritzon J. 1933. Studien über Embryologie
der Familien Crassulaceae und
Saxifragaceae.
– Ph.D. diss, University of Lund, Sweden.
Morf E. 1950. Vergleichend-morphologische
Untersuchungen am Gynoeceum der Saxifragaceen. – Ber. Schweiz. Bot. Ges. 60:
516-590.
Morgan DR, Soltis DE. 1993. Phylogenetic
relationships among members of Saxifragaceae
sensu lato based on rbcL sequence data. – Ann. Missouri Bot. Gard.
80: 631-660.
Nemirovich-Danchenko EN, Lobova TA. 1998. The
seed coat structure in some representatives of the order Hydrangeales. – Bot.
Žurn. 83: 1-9. [In Russian]
Pastre A, Pons A. 1973. Quelques aspects de
la systématique des Saxifragacées à la lumière des données de la
palynologie. – Pollen Spores 15: 117-133.
Patel RN. 1973. Wood anatomy of the
dicotyledons indigenous to New Zealand 2. Escalloniaceae. – New Zealand
J. Bot. 11: 421-434.
Plouvier V. 1956. Sur la presence
d’aspéruloside chez les Escallonia et de dulcitol chez de la
Brexia madagascariensis Thou. (Saxifragacées). – Compt. Rend. Acad.
Sci. 242: 1643-1645.
Ramamonjiarisoa BA. 1980. Comparative anatomy
and systematics of African and Malagasy woody Saxifragaceae
sensu lato. – Ph.D. diss., University of Massachusetts, Amherst,
Massachusetts.
Ramírez C, Sempe J. 1981. Valdivia
gayana als Beispiel einer im subantarkischen Bereich von Südamerika
endemischen Pflanzenart. – Oberhessische Naturwissensch. Zeitschr. 46:
75-80.
Romoleroux K, Freire Fierro A. 2004. Escalloniaceae. – In: Harling
G, Andersson L (eds), Flora of Ecuador 73, Botanical Institute, Göteborg
University, pp. 64-82.
Sax K. 1931. Chromosome numbers in the
ligneous Saxifragaceae.
– J. Arnold Arbor. 12: 198-205.
Schlechter R. 1914. Die Saxifragaceae
Papuasiens. – Engl. Bot. Jahrb. Syst. 52: 118-138.
Schoenagel E. 1931. Chromosomenzahl und
Phylogenie der Saxifragaceen. – Bot. Jahrb. Syst. 64: 266-288.
Scott AJ. 1997. 84. Escalloniacées. – In:
Bosser J, Cadet T, Guého J, Marais W (eds), Flore des Mascareignes – La
Réunion, Maurice, Rodrigues, The Sugar Industry Research Institute,
Mauritius.
Sede SM, Denham SS. 2018. Taxonomic revision
of Escallonia (Escalloniaceae) in Argentina.
– Syst. Bot. 43: 364-369.
Sede SM, Dürnhöfer SI, Morello S, Zapata F.
2013. Phylogenetics of Escallonia (Escalloniaceae) based on plastid
DNA sequence data. – Bot. J. Linn. Soc. 173: 442-451.
Shore BF. 1969. Dioecism in New Zealand Escalloniaceae. – New Zealand
J. Bot. 7: 113-124.
Sleumer H. 1968. Die Gattung
Escallonia (Saxifragaceae).
– Verh. K. Nederl. Akad. Wetensch., Afd. Natuurk., Tweede Sect., 58:
1-146.
Soltis DE, Soltis PS. 1997. Phylogenetic
relationships in Saxifragaceae
sensu lato: a comparison of topologies based on 18S rDNA and rbcL
sequences. – Amer. J. Bot. 84: 504-522.
Soltis DE, Soltis PS, Clegg MT, Durbin M.
1990. rbcL sequence divergence and phylogenetic relationships in Saxifragaceae
sensu lato. – Proc. Natl. Acad. Sci. U.S.A. 87: 4640-4644.
Stern WL. 1974. Comparative anatomy and
systematics of woody Saxifragaceae.
Escallonia. – Bot. J. Linn. Soc. 68: 1-20.
Straka H, Friedrich B. 1988. Familien 65 bis
97. – In: Straka H (ed), Palynologia madagassica et mascarenica,
Steiner-Verl., Wiesbaden, Stuttgart, pp. 1-117.
Swamy BGL. 1954. Comentaria herbaria.
Morpho-taxonomical notes on the Escallonioideae A. Nodal and petiolar
vasculature. – J. Madras Univ., Sect. B, 24: 299-306.
Tomassini L, Foddai S, Nicoletti M, Giuffra
SE, Garcia MR, Bravo FH. 1993. Iridoid glycosides from Escallonia
species. – Biochem. Syst. Ecol. 21: 621-623.
Troncoso AA, San Martin AJ. 1999. Presencia
del genero Escallonia (Magnoliopsida, Escalloniaceae) en el terciario
de Chile central. – Bol. Mus. Nac. Hist. Nat. 48: 29-36.
Wakabayashi M. 1970. On the affinity in Saxifragaceae s.
lato with special reference to the pollen morphology. – Acta Phytotaxon.
Geobot. 24: 128-145. [In Japanese with English summary]
Zapata F. 2010. Phylogenetics and
diversification of Escallonia (Escalloniaceae). – Ph.D.
Thesis, University of Missouri-St. Louis, U.S.A.
Zapata F. 2013. A multilocus phylogenetic
analysis of Escallonia (Escalloniaceae) diversification
in montane South America. – Amer. J. Bot. 100: 526-545.
Zielinski QB. 1955. Escallonia: the
genus and its chromosomes. – Bot. Gaz. 117: 166-172.

