Systematics Cassinopsis (6; C. chapelieri, C. ciliata, C. ilicifolia, C. madagascariensis, C. tinifolia, C. tomentosa; southern and eastern Africa, Madagascar); Nothapodytes (11; East Asia, tropical Asia), Mappia (5; M. angustifolia, M. longipes, M. mexicana, M. multiflora, M. racemosa; southern Mexico, Central America, the Greater Antilles); Alsodeiopsis (11; tropical Africa), Pleurisanthes (7; P. artocarpi, P. brasiliensis, P. emarginata, P. flava, P. howardii, P. parviflora, P. simpliciflora; tropical South America), Merrilliodendron (1; M. megacarpum; the Philippines, islands in western Pacific), Lavigeria (1; L. macrocarpa; Cameroon, Gabon, Congo), Icacina (5; I. claessensii, I. guessfeldtii, I. mannii, I. oliviformis, I. trichantha; tropical Africa), Casimirella (7; C. ampla, C. beckii, C. crispula, C. diversifolia, C. guaranitica, C. lanata, C. rupestris; Central America, tropical South America), Leretia (2; L. cordata, L. racemosa; tropical South America); Mappianthus (1; M. iodoides; southern China), ‘Iodes’ (28; tropical regions in the Old World; paraphyletic; incl. Polyporandra?), Polyporandra (1; P. scandens; East Malesia to New Guinea, Melanesia; in Iodes?); Desmostachys (6; D. brevipes, D. longipes, D. oblongifolius, D. planchonianus, D. tenuifolius, D. vogelii; tropical Africa, Madagascar), Natsiatum (1; N. herpeticum; eastern Himalayas to Southeast Asia), Hosiea (2; H. japonica, H. sinensis; western and central China, Japan), Rhyticaryum (12; East Malesia to New Guinea and western Pacific islands), Sarcostigma (2; S. kleinii, S. paniculata; India to Southeast Asia and Malesia), Miquelia (7; M. assamica, M. caudata, M. celebica, M. kleinii, M. philippinensis, M. reticulata, M. thorelii; tropical Asia), Phytocrene (11; Southeast Asia, Malesia), Stachyanthus (4; S. cuneatus, S. donisii, S. occidentalis, S. zenkeri; tropical Africa), Pyrenacantha (c 35; tropical regions in the Old World)
Unplaced Icacinaceae Natsiatopsis (1; N. thunbergiifolia; Burma, southern Yunnan), Sleumeria (1; S. auriculata; northern Borneo).
Cassinopsis is sister to the remaining Icacinaceae (Byng & al. 2014, Stull & al. 2015)
The Icacina clade is morphologically homogenous and characterized by, e.g., vessel elements with simple perforation plates, lateral pits usually alternate, relatively short vessel elements and fibres, axial parenchyma banded and vasicentric, wood rays multiseriate, high and wide, and variation in the shape of the cambium. Almost all species are lianas.
Sarcostigma has interxylary phloem, few septated libriform fibres occurring together with normal fibres with bordered pits, and sparse biseriate and low multiseriate wood rays. Even the foliar anatomy and the pollen morphology are different from the other lianous Icacinaceae. Natsiatopsis and Sleumeria may be close to Natsiatum (Kårehed 2001, Byng & al. 2014).
 |
|
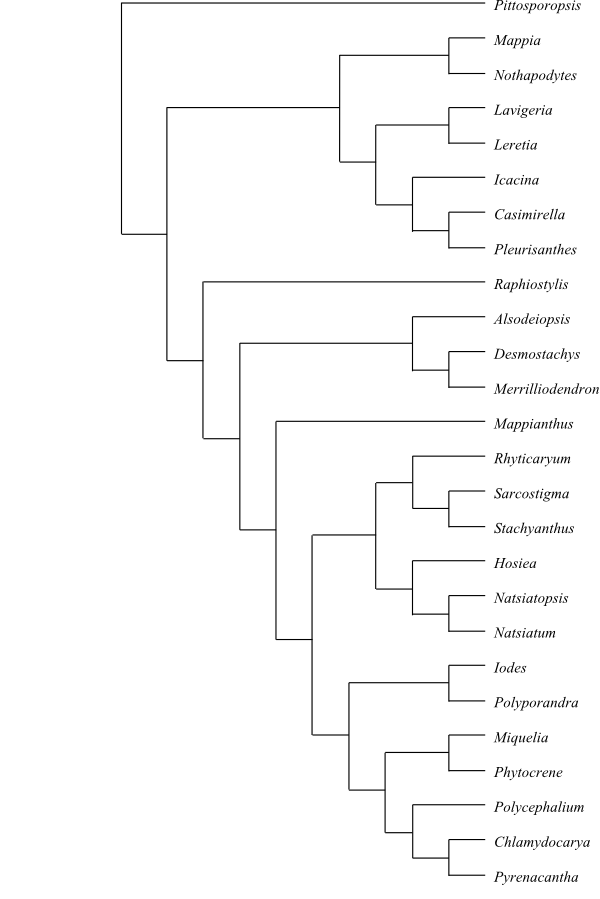
One out of numerous most-parsimonious cladograms of Icacinaceae based on DNA sequence data and morphology (Kårehed 2001). Raphiostylis is usually placed in the Apodytes clade as sister to Apodytes. According to Angulo & al. (2013), Leretia, Icacina and Casimirella form a clade which is more related to other genera than to [Mappia+Nothapodytes]. |
 |
|
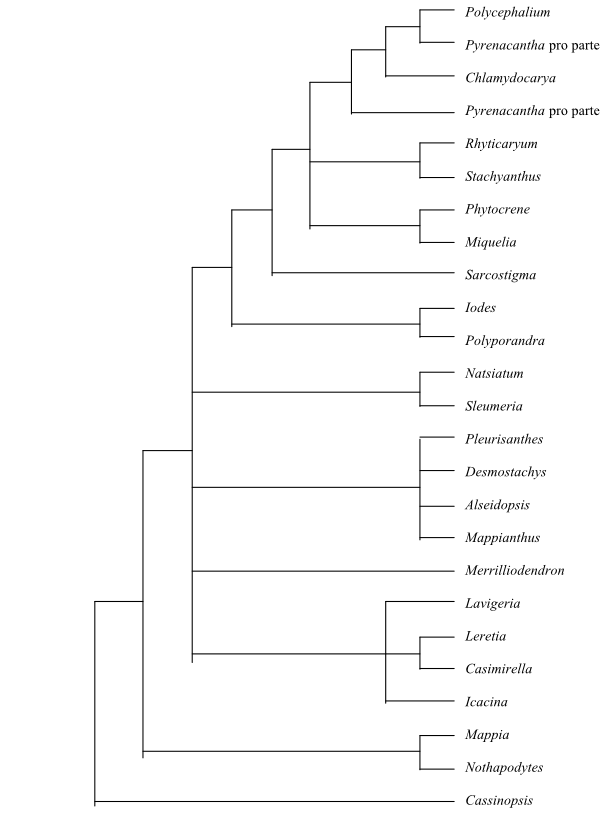
Phylogeny of Icacinaceae based on Byng & al. (2014). |
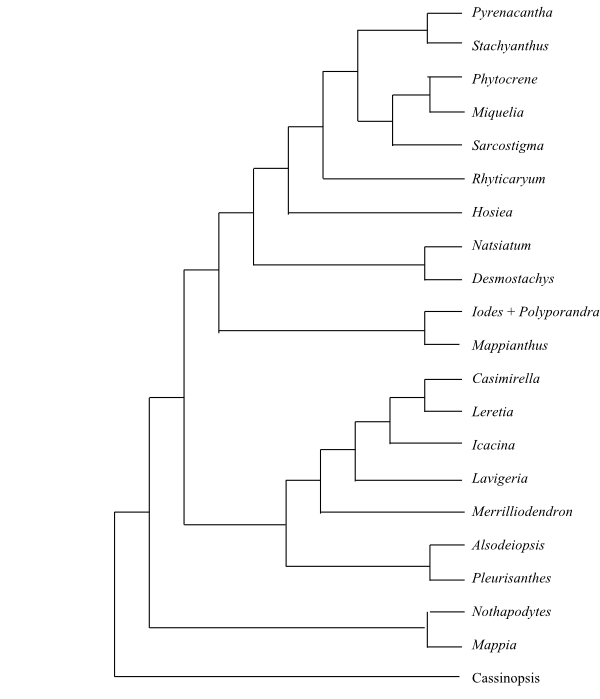 |
Cladogram of Icacinaceae based on DNA sequence data (Stull & al. 2015). |
ONCOTHECACEAE Kobuski ex Airy Shaw |
( Back to Icacinales ) |
Oncothecales Doweld, Tent. Syst. Plant. Vasc.: li. 23 Dec 2001
Genera/species 1/2
Distribution New Caledonia.
Fossils Unknown.
Habit Bisexual, evergreen trees or shrubs.
Vegetative anatomy Phellogen ab initio in outer cortex. Vessel elements with scalariform perforation plates; lateral pits scalariform?, simple pits. Imperforate tracheary xylem elements tracheids or fibre tracheids with bordered pits?, non-septate. Wood rays uniseriate and multiseriate, heterocellular. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal scanty. Secondary phloem in young stems stratified. Sieve tube plastids S type. Nodes usually 5:5, pentalacunar with five leaf traces distally united into invaginated arc (sometimes 3:3, trilacunar with three traces). Asterosclereids present. Parenchyma cells with aggregations of calciumoxalate crystals. Druses abundant. Tanniniferous cells abundant.
Trichomes Hairs absent.
Leaves Alternate (spiral), simple, entire, coriaceous, with convolute? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate. Stomata modified paracytic or anisocytic. Cuticular wax crystalloids? Mesophyll with sclerenchymatous idioblasts. Calciumoxalate druses present. Leaf margin serrate or entire, with small caducous glands on teeth.
Inflorescence Axillary, usually panicle or thyrse. Floral prophylls (bracteoles) triangular, two or three per flower.
Flowers Actinomorphic, small. Hypogyny. Sepals five, with imbricate quincuncial aestivation, persistent, more or less free. Petals five, with imbricate aestivation, caducous, connate. Nectary? Disc absent.
Androecium Stamens five, haplostemonous, antesepalous, alternipetalous. Filaments free, adnate to corolla tube (epipetalous). Anthers basifixed, non-versatile, disporangiate (dithecal), extrorse, longicidal (dehiscing by longitudinal slits); connective in Oncotheca balansae (not in O. humboldtiana) acute and incurved above gynoecium. Tapetum secretory? Staminodia absent.
Pollen grains Microsporogenesis simultaneous? Pollen grains (2–)3-colporate, shed as monads, bicellular? at dispersal. Exine tectate, with columellate infratectum, perforate, smooth.
Gynoecium Pistil composed of five syncarpous and connate antepetalous carpels. Ovary superior, quinquelocular, with five ridges, apically somewhat quinquelobate (with lobes incompletely closed). Stylodia five, free, recurved, conduplicate. Stigmas ventral, punctate, type? Pistillodium absent.
Ovules Placentation apical to axile. Ovules usually two (sometimes one) per carpel, anatropous, pendulous, epitropous, unitegmic, crassinucellar. Funicle long. Integument four to seven cell layers thick. Megagametophyte monosporous, Polygonum type? Endosperm development nuclear? Endosperm haustoria? Embryogenesis?
Fruit A two- to five-seeded drupe with thick quinquelocular pyrene and persistent calyx.
Seeds Aril absent. Testa thin. Exotesta? Endotesta? Perisperm not developed. Endosperm copious. Embryo straight, cylindrical, well differentiated, with chlorophyll? Hypocotyl long. Cotyledons usually two (rarely three), short. Germination?
Cytology n = 25
DNA
Phytochemistry Virtually unknown. Tannins present.
Use Unknown.
Systematics Oncotheca (2; O. balansae, O. humboldtiana; New Caledonia).
Literature
Allen SE, Stull GW, Manchester SR. 2013. Icacinaceae from the Eocene of western North America. – Am. J. Bot. 102: 725-744.
Angulo DF, Stefano RD de, Stull GW. 2013. Systematics of Mappia (Icacinaceae), an endemic genus of tropical America. – Phytotaxa 116: 1-18.
Baas P. 1973. Epidermal leaf characters of the Malesian Icacinaceae. – Acta Bot. Neerl. 22: 329-359.
Baas P. 1974. Stomatal types in Icacinaceae. Additional observations on genera outside Malesia. – Acta Bot. Neerl. 23: 193-200.
Baas P. 1975. Vegetative anatomy and the affinities of Aquifoliaceae, Sphenostemon, Phelline, and Oncotheca. – Blumea 22: 311-407.
Bailey IW, Howard RA. 1941a. The comparative morphology of the Icacinaceae I. Anatomy of the node and internode. – J. Arnold Arbor. 22: 125-132.
Bailey IW, Howard RA. 1941b. The comparative morphology of the Icacinaceae II. Vessels. – J. Arnold Arbor. 22: 171-187.
Bailey IW, Howard RA. 1941c. The comparative morphology of the Icacinaceae III. Imperfect tracheary elements and xylem parenchyma. – J. Arnold Arbor. 22: 432-442.
Bailey IW, Howard RA. 1941d. The comparative morphology of the Icacinaceae IV. Rays of the secondary xylem. – J. Arnold Arbor. 22: 556-568.
Baillon HE. 1891. Sur le nouveau genre Oncotheca. – Bull. Mens. Soc. Linn. Paris 2: 931-932.
Bâthie HP de la. 1952. Icacinacées (Icacinaceae). – In: Humbert H (ed), Flore de Madagascar et des Comores 119, Typographie Firmin-Didot et Cie, Paris.
Bautista PB, De Andrade TAP. 1975. O pólen em plantas da Amazônia V. Contribuição ao estudo da familia Icacinaceae. – Bol. Mus. Paraense “Emilio Goeldi”, N. S., Bot. 47: 1-11.
Braga de Oliveira A. 1995. Terpenoids from Amazonian Icacinaceae. – In: Chemistry of the Amazon, ACS Symposium Ser., Vol. 588, pp. 99-115.
Breteler FJ, Villiers J-F. 2000. Novitates Gabonenses 39. Une nouvelle espèce de Pyrenacantha (Icacinaceae) du Gabon. – Adansonia, sér. III, 22: 201-204.
Byng JW, Bernardini B, Joseph JA, Chase MW,
Utteridge TMA. 2014. Phylogenetic relationships of Icacinaceae focusing on the
vining genera. – Bot. J. Linn. Soc. 176: 277-294.
Cameron KM. 2003. On the phylogenetic position of the New Caledonian endemic families Paracryphiaceae, Oncothecaceae, and Strasburgeriaceae: a comparison of molecules and morphology. – Bot. Rev. 68: 428-443.
Capuron R. 1970. Notes sur les Icacinaceae. – Adansonia, n. s., 10: 507-510.
Carpenter CS. 1975. The morphology and relationships of Oncotheca balansae. – Ph.D. diss., Master of Arts, University of North Carolina, Chapel Hill, North Carolina.
Carpenter CS, Dickison WC. 1976. The morphology and relationships of Oncotheca balansae. – Bot. Gaz. 137: 141-153.
Dahl AO. 1952. The comparative morphology of the Icacinaceae VI. The pollen. – J. Arnold Arbor. 33: 252-295.
Dahl AO. 1955. The pollen morphology of several genera excluded from the family Icacinaceae. – J. Arnold Arbor. 36: 159-163.
Dickison WC. 1982. Vegetative anatomy of Oncotheca macrocarpa: a newly described species of Oncothecaceae. – Bull. Mus. Natl. Hist. Nat. (Paris), sér. IV, sect. B, Adansonia 3: 177-181.
Dickison WC. 1986. Further observations on the floral anatomy and pollen morphology of Oncotheca (Oncothecaceae). – Brittonia 38: 249-259.
Engler A. 1896. Icacinaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(5), W. Engelmann, Leipzig, pp. 233-257, 459-460; Engler A. 1897. Nachträge zu III(5), pp. 225-227.
Fagerlind F. 1945. Bau des Gynöceums, der Samenanlage, und des Embryosackes bei einigen Repräsentanten der Familie Icacinaceae. – Svensk Bot. Tidskr. 39: 346-364.
Graebner IB, Mostardeiro MA, Ethur EM, Burrow RA, Dessoy EC, Morel AF. 2000. Diterpenoids from Humirianthera ampla. – Phytochemistry 53: 955-959.
Guillaumin A. 1938. Observations morphologiques et anatomiques sur le genre Oncotheca. – Rev. Gén. Bot. 50: 629-635.
Guymer GP. 1984. Icacinaceae. – In: George AS (ed), Flora of Australia 22, Australian Government Publ. Service, Canberra, pp. 204-212.
Haron NW, Ping ST. 1997. Distribution and taxonomic significance of flavonoids in the Olacaceae and Icacinaceae. – Biochem. Syst. Ecol. 25: 265-263.
Heads M. 2010. The endemic plant families and the palms of New Caledonia: a biogeographical analysis. – J. Biogeogr. 37: 1239-1250.
Heintzelmann CE, Howard RA. 1948. The comparative morphology of the Icacinaceae V. The pubescence and the crystals. – Amer. J. Bot. 35: 42-52.
Howard RA. 1940. Studies of the Icacinaceae I. Preliminary taxonomic notes. – J. Arnold Arbor. 21: 461-489.
Howard RA. 1942a. Studies of the Icacinaceae II. Humirianthera, Leretia, Mappia and Nothapodytes, valid genera of the Icacineae. – J. Arnold Arbor. 23: 55-78.
Howard RA. 1942b. Studies of the Icacinaceae III. A revision of Emmotum. – J. Arnold Arbor. 23: 479-494.
Howard RA. 1942c. Studies of the Icacinaceae IV. Considerations of the New World genera. – Contr. Gray Herb. Harvard Univ. 142: 3-60.
Howard RA. 1943. Studies of the Icacinaceae VIII. Brief notes of some Old World genera. – Lloydia 6: 144-154.
Howard RA. 1992. A revision of Casimirella, including Humirianthera (Icacinaceae). – Brittonia 44: 166-172.
Kadereit JW, Bittrich V (eds). 2016. The families and genera of vascular plants XIV. Flowering plants – eudicots – Aquifoliales, Boraginales, Bruniales, Dipsacales, Escalloniales, Garryales, Paracryphiales, Solanales (except Convolvulaceae), Icacinaceae, Metteniusaceae, Vahliaceae. Springer, 412 pp.
Kaplan MAC, Ribeiro J, Gottlieb OR. 1991. Chemographical evolution of terpenoids in Icacinaceae. – Phytochemistry 30: 2672-2676.
Kårehed J. 2001. Multiple origin of the tropical forest tree family Icacinaceae. – Amer. J. Bot. 88: 2259-2274.
Kårehed J. 2002. Not just hollies – the expansion of Aquifoliales. – In: Evolutionary studies in asterids emphasising euasterids II, Ph.D. diss., Acta Universitatis Upsaliensis, Uppsala, Sweden, pp. 1-14.
Labat J-N, El-Achkar E, Rabevohitra R. 2006. Révision synoptique du genre Pyrenacantha (Icacinaceae) à Madagascar. – Adansonia, sér. III, 28: 399-404.
Lens F, Kårehed J, Baas P, Jansen S, Rabaey D, Huysmans S, Hamann T, Smets E. 2008. The wood anatomy of polyphyletic Icacinaceae s.l., and their relationships within asterids. – Taxon 57: 525-552.
Lobreau-Callen D. 1972. Le pollen des Icacinaceae I. Atlas (1). – Pollen Spores 14: 345-388.
Lobreau-Callen D. 1973. Le pollen des Icacinaceae II. Observations en microscopie électronique, corrélations, conclusions. – Pollen Spores 15: 47-89.
Lobreau-Callen D. 1980. Caractères comparés du pollen des Icacinaceae et des Olacaceae. – Adansonia, sér. II, 20: 29-89.
Loesener T. 1942. Aquifoliaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 36-86.
Lorence A, Nessler CL. 2004. Camptothecin, over four decades of surprising findings. – Phytochemistry 65: 2735-2749.
Lucas GL. 1968. Icacinaceae. – In: Milne-Redhead E, Polhill RM (eds), Flora of tropical East Africa, Crown Agents for Overseas Governments and Administrations, London, pp. 1-18.
McPherson G, Morat P, Veillon JM. 1982. Existence d’une deuxième espèce appartenant au genre Oncotheca endémique de la Nouvelle-Calédonie et nouvelle données concernant les Oncothécacées. – Bull. Mus. Natl. Hist. Nat. (Paris), sér. IV, sect. B, Adansonia 3: 305-311.
Mauritzon J. 1936. Embryologische Angaben über Stackhousiaceae, Hippocrateaceae und Icacinaceae. – Svensk Bot. Tidskr. 30: 541-550.
Mendes EJ. 1963. 50. Icacinaceae. – In: Exell AW, Fernandes A, Wild H (eds), Flora Zambesiaca 2 (Part 1), Crown Agents for Oversea Governments and Administrations, London, pp. 340-351.
Miers J. 1852. Observations on the affinities of the Icacinaceae. – Ann. Mag. Nat. Hist. 2nd ser. 18: 218-226.
Perrier de la Bâthie H. 1944. Révision des Icacinacées de Madagascar et des Comores. – Mém. Mus. Natl. Hist. Nat. 18: 289-308.
Pigg KB, Manchester SR, DeVore ML. 2008. Fruits of Icacinaceae (tribe Iodeae) from the Late Paleocene of western North America. – Amer. J. Bot. 95: 824-832.
Pittier H. 1925. Árboles y arbustos nuevos de Venezuela. – Bol. Sci. Técn. Mus. Comercial Venezuela 1: 45-47.
Potgieter MJ, Wyk AE van. 1994a. Fruit structure of the genus Cassinopsis Sond. (Icacinaceae) in Africa. – South Afr. J. Bot. 60: 117-122.
Potgieter MJ, Wyk AE van. 1999. Leaf anatomy of the southern African Icacinaceae and its taxonomic significance. – South Afr. J. Bot. 65: 153-162.
Puri SC, Verma V, Amna T, Qazi GN, Spiteller M. 2005. An endophytic fungus from Nothapodytes foetida that produces camptothecin. – J. Nat. Prod. 68: 1717-1719.
Rankin BD, Stockey RA, Beard G. 2008. Fruits of Icacinaceae from the Eocene Appian Way locality of Vancouver Island, British Columbia. – Intern. J. Plant Sci. 169: 305-314.
Rasoanaivo P, Ratsimmamanga-Urverg S, Messana I, DeVicente Y, Galeffi C. 1990. Cassinopin, a kaempferol trirhamnoside from Cassinopsis madagascariensis. – Phytochemistry 29: 2040-2043.
Shiklina IA. 1977. Comparative anatomy of the wood of the genus Oncotheca Baill. (Theales). – Bot. Žurn. 62: 1273-1275. [In Russian]
Sleumer H. 1942. Icacinaceae. – In: Engler A (†), Harms H, Mattfeld J (eds), Die natürlichen Pflanzenfamilien, 2. Aufl., Bd. 20b, W. Engelmann, Leipzig, pp. 322-396.
Sleumer H. 1969. Materials towards the knowledge of the Icacinaceae of Asia, Malesia, and adjacent areas. – Blumea 17: 181-264.
Sleumer H. 1971. Icacinaceae. – In: Steenis CGGJ van (ed), Flora Malesiana I, 7, Wolters-Noordhoff, Groningen, pp. 1-87.
Spichiger R, Savolainen V, Manen J-F. 1993. Systematic affinities of Aquifoliaceae and Icacinaceae from molecular data analysis. – Candollea 48: 459-464.
Staveren MGC van, Baas P. 1973. Epidermal leaf characters of Malesian Icacinaceae. – Acta Bot. Neerl. 22: 329-359.
Stull GW, Moore RW, Manchester SR. 2011. Fruits of Icacinaceae from the Eocene of southeastern North America and their biogeographic implications. – Intern. J. Plant Sci. 172: 935-947.
Stull GW, Herrera F, Manchester SR, Jaramillo C, Tiffney BH. 2012. Fruits of an “Old World” tribe (Phytocreneae; Icacinaceae) from the Paleogene of North and South America. – Syst. Bot. 37: 784-794.
Stull GW, Stefano RD de, Soltis DE, Soltis PS. 2015. Resolving basal lamiid phylogeny and the circumscription of Icacinaceae with a plastome-scale data set. – Amer. J. Bot. 102: 1794-1813.
Teo SP, Haron NW. 1999. Anatomical studies in West Malaysian Icacinaceae. – Aust. Syst. Bot. 11: 729-738.
Utteridge TMA, Nagamasu H, Teo SP, White LC, Gasson P. 2005. Sleumeria (Icacinaceae): a new genus from northern Borneo. – Syst. Bot. 30: 635-643.
Villiers J-F. 1973. Icacinaceae. – In: Aubréville A, Leroy J-F (eds), Flore de Gabon 20, Muséum National d’Histoire Naturelle, Paris, pp. 3-100.
Villier J-F. 1980. Icacinaceae. – In: Aubréville A, Leroy J-F (eds), Flore de la Nouvelle Calédonie 9, Muséum National d’Histoire Naturelle (Paris), pp. 159-174.
Wagner R. 1923. Über Vorkommnisse von Domatien bei Icacinaceen. – Anz. Akad. Wiss. Wien., Math.-Naturw. Kl. 60: 189-193.