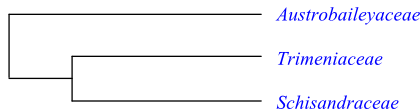
Phylogeny of Schisandrales based on DNA sequence data.
[Schisandrales+[[Chloranthaceae+Magnoliidae]+[Liliidae+[Ceratophyllum+Tricolpatae]]]]
Illiciales Hu ex Cronquist, Integr. Syst. Class. Fl. Pl.: 94. 10 Aug 1981; Austrobaileyales Takht. ex Reveal in Novon 2: 238. 13 Oct 1992; Illicianae Doweld, Tent. Syst. Plant. Vasc.: xxiii. 23 Dec 2001;
Habit Bisexual, monoecious, andromonoecious, polygamomonoecious, dioecious or polygamodioecious, usually evergreen (rarely deciduous) trees, shrubs, lianas or climbing shrubs. Aromatic (in Austrobaileya with foul-smelling flowers).
Vegetative anatomy Mycorrhiza? Phellogen ab initio superficial. Primary stem with continuous vascular cylinder, pseudosiphonostele, or with separate vascular bundles. Secondary lateral growth present. Vessel elements often tracheoid, with scalariform or simple perforation plates; lateral pits scalariform, opposite or alternate, simple or bordered pits. Imperforate tracheary xylem elements tracheids or fibre tracheids with simple or bordered pits, usually non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty, unilateral or banded, or often absent. Sieve tube plastids S type. Nodes 1:1–3, unilacunar with one to three leaf traces. Sclereids fibre-like, with crystals or branched asterosclereids. Cortex, pith and phloem sometimes with mucilage cells. Outer cortex and phloem sometimes with idioblasts containing ethereal oils. Cortex sometimes with calciumoxalate as crystal sand.
Trichomes Hairs unicellular, multicellular, uniseriate, or absent.
Leaves Usually alternate (spiral; sometimes opposite), simple, entire, often coriaceous, with supervolute, involute or conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate, brochidodromous. Stomata laterocytic or paracytic (sometimes stephanocytic, rarely anomocytic). Abaxial side of lamina with radiate-striate cuticular patterns surrounding secretory cells (absent in Trimenia). Cuticular wax crystalloids as irregular platelets, dominated by nonacosan-10-ol. Epidermis cells often silicified. Ethereal oil cell complexes present on abaxial surface. Mesophyll with spherical idioblasts containing mucilage or ethereal oils. Asterosclereids present. Leaf margin usually entire or sometimes serrate and teeth then with persistent opaque swollen hood-shaped structure, into which a vein is running (chloranthoid or rarely monimioid teeth).
Inflorescence Flowers usually solitary (sometimes two or more together) axillary (rarely terminal; sometimes panicles or racemes).
Flowers Actinomorphic. Hypogyny. Tepals (five to) twelve to 38, initially spiral, later usually whorled, in one to three series, with imbricate aestivation, free; outer tepals bracteolar to sepaloid; inner tepals sepaloid to petaloid (innermost tepals sometimes staminodial). Nectaries absent. Disc absent.
Androecium Stamens four to more than 80, initially spiral, later usually whorled, in one to three whorls. Filaments subulate or flattened (sometimes foliaceous), free or entirely to only at base connate; free from tepals. Anthers basifixed, non-versatile, tetrasporangiate (microsporangia rarely embedded in adaxial side of connective), introrse, latrorse or extrorse, longicidal (dehiscing by longitudinal slits). Tapetum secretory, with (uninucleate or) binucleate cells. Staminodia three to 22, extrastaminal (sometimes petaloid), or absent.
Pollen grains Microsporogenesis usually simultaneous (rarely successive). Pollen grains globose, hexacolpate, tricolpate or tricolpoidate (modified monosulcate via trichotomosulcate), with extra-apertural endexine, sometimes heteropolar, colpi displaced according to Garside’s rule (rarely inaperturate, disulculate or polyforate), usually shed as monads (rarely tetrads), bicellular at dispersal. Exine usually semitectate (rarely tectate), with usually columellate (rarely granular) infratectum, reticulate or perforate, regulate, scabrate, verrucate or psilate.
Gynoecium Carpels (five to) seven to c. 100 (to more than 300), initially spiral, later usually whorled, free; carpel conduplicate, usually ascidiate (sometimes partially plicate), carpellary margins postgenitally occluded by secretion or postgenitally fused in periferal part (incompletely fused at anthesis). Ovary superior, unilocular (apocarpy or pseudomonomery). Style short or absent. Stigma long, curved, distributed along upper side of style or as two longitudinal adaxial decurrent stigmatic areas, covered by mucilaginous layer (extragynoecial mucilaginous compitum facilitating pollination in multiple carpels from pollen grains present on one stigmatic zone), papillate or non-papillate, Dry or Wet type. Pistillodium usually absent (male flowers sometimes with pistillodium).
Ovules Placentation lateral (adaxial), subbasal-lateral or apical. Ovules one to five (to 14) per carpel, usually anatropous (rarely campylotropous?), pendulous or ascending, apotropous, bitegmic, crassinucellar. Micropyle usually bistomal. Archespore unicellular to multicellular. Nucellar cap sometimes present. Megagametophyte monosporous (megaspore chalazal), quadrinucleate and quadricellular with two synergids and one egg cell at micropylar pool and a uninucleate haploid central cell, Nuphar/Schisandra type (sometimes Oenothera type; occasionally disporous?, with several megasporocytes); the two nuclei after first division present at micropylar pool; egg cell differentiating from one of three micropylar cells after second division. Antipodal cells absent. Endosperm cells diploid. One sperm nucleus and one polar nucleus fusing at second fertilization. Endosperm development cellular. Endosperm haustoria? Embryogenesis onagrad or asterad.
Fruit An assemblage of berry-like drupes or a multifolliculus consisting of one-seeded follicles (sometimes a berry).
Seeds Aril absent. Seed coat exotestal-mesotestal. Exotesta palisade. Mesotestal cells sclerotic. Endotesta unspecialized (sometimes lignified). Tegmen unspecialized. Perisperm usually not developed (rarely well developed). Endosperm copious, oily (sometimes starchy as well; rarely ruminate). Embryo small, straight, well differentiated, without chlorophyll. Cotyledons two. Germination phanerocotylar.
Cytology x = 7, 8, 11–13
DNA 12 bp deletion (representing four amino acids) in exon 5’ of PI gene (normal character state of all angiosperms other than Amborella and Nymphaeales.
Phytochemistry 5-O-methylflavonols (kaempferol, quercetin, myricetin), flavones, cyanidin, monoterpenes, tetracyclic triterpenes, spirosesquiterpenes and other sesquiterpenes, sesquiterpene lactones, allylphenols (anisoxide etc.), prodelphinidins, piptoside, tiglic acid and angelic acid in the form of esters with dibenzocyclo-octanoids, germacrane-like substances, kadsurin A, myristicin, lignans (podophyllotoxin, veraguensin, austrobailignan, calopiptin), and neolignans present. Ellagic acid and benzylisoquinoline alkaloids not found.
Systematics Schisandrales and Nymphaeales have a quadrinucleate quadricellular megagametophyte, at maturity containing a tricellular egg apparatus and a central cell with a single haploid polar nucleus (Friedman & Ryerson 2008). The two nuclei resulting from the first mitotic division of the single functional megaspore remain at the micropylar pole, and a second division produces four micropylar megagametophyte nuclei. The egg cell develops from one of these nuclei during the differentiation of the three-celled egg apparatus, whereas the fourth cell forms the nucleus of the central cell. Thus, the endosperm becomes diploid instead of triploid.
Schisandraceae and Trimenia share several characteristics not present in Austrobaileya, e.g. lobate outer integument, palisade exotesta and frequent presence of mucilage idioblasts. A synapomorphy of all three clades are the oil cell complexes occurring at least on the abaxial leaf epidermis. According to Carpenter (2006), these structures are homologous with, e.g., (actinocytic/stephanocytic) stomata in Amborella and Trimenia and the hydropotes in Nymphaeales.

|
Phylogeny of Schisandrales based on DNA sequence data. |
AUSTROBAILEYACEAE (Croizat) Croizat |
Genera/species 1/2
Distribution Northeastern Queensland (Australia).
Fossils Unknown.
Habit Bisexual, evergreen, climbing shrubs or lianas. The flowers with an odour reminiscent of decaying fish.
Vegetative anatomy Phellogen superficial? Primary stem with separate vascular bundles. Secondary lateral growth present. Vessel elements with scalariform perforation plates; lateral pits scalariform, opposite or alternate, bordered pits. Imperforate tracheary xylem elements septate or non-septate? tracheids or septate fibre tracheids with bordered pits (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma paratracheal scanty or unilateral. Sieve tube plastids S type, with numerous extremely large starch grains. Sieve tube cells with non-dispersive protein bodies. Nodes 1:2, unilacunar with two leaf traces. Outer cortex and phloem with idioblasts containing ethereal oils. Cortex with calciumoxalate as crystal sand.
Trichomes Hairs absent.
Leaves Opposite, simple, entire, coriaceous, with conduplicate ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate, brochidodromous. Stomata paracytic or anomocytic. Cuticular wax crystalloids as irregular platelets (chemically dominated by nonacosan-10-ol?). Mesophyll with idioblasts containing ethereal oils, and tanniniferous cells. Ethereal oil cell complexes present only on abaxial surface. Leaf margin entire.
Inflorescence Flowers usually axillary (sometimes terminal), usually solitary (rarely few-flowered botryoid). Floral prophylls (bracteoles) grading into tepals.
Flowers Actinomorphic. Hypogyny. Tepals 19–24, with imbricate aestivation, spiral, free; outer tepals sepaloid, grading inwards into petaloid tepals. Nectaries absent. Disc absent.
Androecium Stamens six to eleven, foliaceous, papillate, spiral, free, not differentiated into filaments and anthers, grading inwards into staminodia. Microsporangia four, embedded in adaxial side of connective (as young marginal), introrse, longicidal (dehiscing by longitudinal slits). Tapetum secretory?, with uninucleate or binucleate cells. Staminodia nine to 16, intrastaminal. Stamens and staminodia strongly odorous (like decaying fish).
Pollen grains Microsporogenesis simultaneous (with rudimentary cell plate). Pollen grains globose, anasulcate (monosulcate?), shed as monads, bicellular at dispersal. Exine tectate, with columellate infratectum, perforate, scabrate (rugulate?).
Gynoecium Carpels (six to) nine (to 14), spiral, free; carpel strongly ascidiate, stipitate, occluded by secretion (carpel margins not fused); mucilaginous extragynoecial compitum present. Ovary superior, unilocular (apocarpy). Style short, with secretory stigmatic canal. Stigma bilobate, decurrent, papillate, Wet type, at anthesis covered with mucilage. Pistillodium absent.
Ovules Placentation lateral (adaxial). Ovules (four to) six to eight (to 14) per carpel, anatropous, apotropous, bitegmic, crassinucellar. Micropyle usually bistomal (sometimes endostomal). Outer integument five to seven cell layers thick. Inner integument two or three cell layers thick. Nucellar cap present. Chalaza perichalazal. Megagametophyte monosporous, quadrinuclear, Nuphar/Schisandra type. Endosperm development cellular? Endosperm haustoria? Embryogenesis?
Fruit A berry, often large.
Seeds Aril absent. Seed coat testal. Testa multiplicative, vascularized. Sarcotesta (developed from outer testal layers) present. Mesotesta partly sclerotic with outer mesotestal cell walls lignified, partially as sarcotesta (inner mesotestal cells). Tegmen unspecialized, crushed. Perisperm not developed. Endosperm copious, starchy, ruminate. Embryo small, straight, chlorophyll? Cotyledons two. Germination phanerocotyl.
Cytology n = 22, 23
DNA
Phytochemistry Insufficiently known. Proanthocyanidins (prodelphinidins?), tiglic acid, austrobailignan, and neolignans present. Alkaloids not found. Myricetin?
Use Unknown.
Systematics Austrobaileya (2; A. scandens: northeastern Queensland, southeastern New South Wales; A. maculata: northeastern Queensland).
Austrobaileya is sister to [Trimenia+Schisandraceae].
SCHISANDRACEAE Blume |
Illiciaceae Bercht. et J. Presl, Přir. Rostlin 2(72): 288. 1825 [‘Illicieae’], nom. cons.; Kadsuraceae Radogizky in Žurn. Sadevodstra 6: 8, 10. 2 Dec 1849 [‘Kadzuraceae’]
Genera/species 3/c 80
Distribution Sri Lanka, Simla to Bhutan, Assam and Burma, East Asia to Japan and Manchuria, Southeast Asia, West Malesia, southeastern North America and southern Mexico; Illicium also in the Greater Antilles (Cuba, Hispaniola, Jamaica, etc.).
Fossils Illiciospermum pusillum consists of fossil seeds from the Cenomanian to Turonian (Late Cretaceous) of Kazakhstan. They are similar to seeds of Illicium and the outer integument has a slit-like micropylar opening situated on a bulge dorsal to the hilum and transversely directed to the plane of symmetry. Hexacolpate fossil pollen grains are known from the Maastrichtian of California. Fossil Schisandraceae are more frequent in Cenozoic layers.
Habit Bisexual (Illicium), or monoecious or dioecious (Schisandra, Kadsura), usually evergreen (rarely deciduous) trees or shrubs (Illicium), or lianas or climbing shrubs (Schisandra, Kadsura). Aromatic.
Vegetative anatomy Phellogen ab initio superficial. Primary stem with continuous vascular cylinder, (pseudo)siphonostele. Vessel elements (in Illicium tracheoid) with scalariform or simple perforation plates; lateral pits scalariform or opposite (sometimes alternate), simple or bordered pits. Imperforate tracheary xylem elements tracheids with simple or bordered pits, non-septate (also vasicentric tracheids). Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma apotracheal diffuse, or paratracheal scanty or banded, or absent. Secondary lateral growth present. Sieve tube plastids S type, with ten to twenty starch grains. Nodes 1:1 (Illicium) or 1:3 (Schisandra, Kadsura), unilacunar with one or three leaf traces, respectively. Sclereids fibre-like, with crystals in secondary walls (Schisandra, Kadsura) or branched asterosclereids (Illicium). Cortex, pith and phloem often with mucilage cells.
Trichomes Hairs multicellular uniseriate, eglandular, or absent.
Leaves Alternate (spiral), simple, entire, often coriaceous, with supervolute (Illicium) or involute (Schisandra, Kadsura) ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate. Venation pinnate. Stomata usually paracytic (rarely anomocytic), in Illicium with dumb-bell-shaped guard cells and two to four parallel subsidiary cells, in Schisandra and Kadsura also modified laterocytic. Cuticular wax crystalloids as irregular platelets, dominated by nonacosan-10-ol. Epidermal cells often silicified, usually with mucilaginous idioblasts? Mesophyll with spherical idioblasts with mucilage or ethereal oils. Asterosclereids present. Leaf surface often gland-dotted. Ethereal oil cell complexes present on abaxial surface. Cuticle of abaxial surface radiate-striate around secretory cells. Leaf margin usually entire (sometimes serrate); leaf teeth with opaque persistent swollen hood-shaped structure, into which a vein is running (chloranthoid teeth; in Schisandra, Kadsura and some species of Illicium).
Inflorescence Flowers axillary to almost terminal, usually solitary (in Schisandra and Kadsura sometimes few-flowered raceme). Floral prophyll (bracteole) in Schisandra adaxial or lateral?
Flowers Actinomorphic. Hypogyny. Tepals in Illicium (seven to) twelve to 30 (to 33), in Schisandra and Kadsura five to 15 (to 24), initially spiral, later whorled, in one to three series, free; outer tepals bracteolar to sepaloid; inner tepals sepaloid to petaloid (innermost tepals in Illicium sometimes staminodial). Nectaries absent. Disc absent.
Androecium Stamens in Illicium four to c. 40 (occasionally to c. 50), in Schisandra and Kadsura four to c. 80 (to more than 300), initially spiral, later whorled, in one to three series. Filaments subulate or flattened, free (Illicium), or entirely to only at base connate (Schisandra, Kadsura); free from tepals. Anthers basifixed, non-versatile, tetrasporangiate, introrse (Illicium), latrorse (Kadsura) or extrorse (Schisandra), longicidal (dehiscing by longitudinal slits); connective in Illicium somewhat prolonged, in Schisandra and Kadsura wide. Tapetum secretory, with binucleate cells. Staminodia in Illicium often petaloid, extrastaminal; in some species of Kadsura three to 22, intrastaminal.
Pollen grains Microsporogenesis simultaneous. Pollen grains globose, in Illicium tricolpate or tricolpoidate (i.e. modified monosulcate via trichotomosulcate); pollen grains in Schisandra and Kadsura heteropolar (syncolpate at distal pole), usually hexacolpate (rarely tricolpate; colpi displaced c. 60o relative to pollen grains in Tricolpatae, hence according to Garside’s rule), shed as monads, bicellular at dispersal. Exine semitectate, with columellate infratectum, reticulate with tall muri, psilate.
Gynoecium Carpels in Illicium (five to) seven to 15 (to 21), in Schisandra and Kadsura twelve to c. 100 (to more than 300), initially spiral, later whorled in one series (Illicium), or spiral (Schisandra, Kadsura), free; carpel usually ascidiate (in Illicium sometimes ascoplicate), in Kadsura and Schisandra occluded by secretion (carpel margins not fused); carpel in Illicium postgenitally closed in periferal part, incompletely closed at anthesis; mucilaginous extragynoecial compitum present. Ovary superior, unilocular (apocarpy). Stylodia short, thin or conical. Stigmas long, curved, present along upper side of stylodium, non-papillate, Dry type (Illicium), or as two longitudinal adaxial decurrent stigmatic areas, papillate, Wet type (Schisandra, Kadsura), covered by mucilaginous layer. Pistillodia?
Ovules Placentation submarginal (lateral, adaxial; Schisandra, Kadsura), or subbasal-lateral (Illicium). Ovule one (Illicium), or ovules two or sometimes three (Schisandra), or usually two to five (rarely up to eleven; Kadsura) per carpel, usually anatropous (rarely campylotropous?), pendulous (Schisandra, Kadsura) or ascending (Illicium), bitegmic, crassinucellar. Micropyle endostomal (always?). Outer integument five to seven cell layers thick. Inner integument at least three cell layers thick. Archespore at least three-celled (Illicium). Megagametophyte monosporous, quadrinuclear, Nuphar/Schisandra type (in Illicium sometimes disporous?, with several megasporocytes). Endosperm development cellular. Endosperm haustoria? Embryogenesis onagrad or asterad.
Fruit An assemblage of usually two-seeded berry-like drupes on expanded (Kadsura) or elongated (Schisandra) receptacle, or a multifolliculus consisting of one-seeded follicles (Illicium).
Seeds Aril absent. Illicium with seeds having a circular cap. Seed coat exotestal. Testa in Illicium sometimes multiplicative. Exotesta palisade and sclerenchymatous, in Illicium with sinuous outer anticlinal cell walls. Mesotestal cells sclerotic. Endotesta in Illicium unspecialized, in some species of Schisandra lignified. Tegmen unspecialized, often crushed. Perisperm not developed. Endosperm copious, oily (and starchy?). Embryo small, straight, well differentiated, without chlorophyll (Illicium). Cotyledons two. Germination phanerocotylar.
Cytology x = 13, 14 (in Schisandra and Kadsura also x = 7)
DNA 12 bp deletion (representing four amino acids) in exon 5’ of PI gene (normal character state of all angiosperms other than Amborella and Nymphaeales.
Phytochemistry 5-O-methylflavonols (kaempferol, quercetin, myricetin), cyanidin, monoterpenes, tetracyclic triterpenes (Illicium), sesquiterpenes (e.g. a spirosesquiterpene in Kadsura and Schisandra), sesquiterpene lactones, allylphenols (anisoxide etc., in Illicium), proanthocyanidins (prodelphinidins), tiglic acid and angelic acid in the form of esters with dibenzocyclo-octanoids, germacrane-like substances, kadsurin A, myristicin, lignans (podophyllotoxin, in Illicium), and neolignans (Schisandra, Kadsura) present. Flavones and ellagic acid not found. Aluminium accumulation occurring in Illicium.
Use Ornamental plants, medicinal plants, spices and flavours (Illicium verum etc.).
Systematics Illicium (42; India, Southeast and East Asia, eastern North America, Mexico, the West Indies); Kadsura (16; India, Southeast and East Asia, West Malesia, the Moluccas), Schisandra (23; eastern Asia to Manchuria, Sakhalin and Japan, southeastern North America).
Schisandraceae are sister to Trimenia (Trimeniaceae). There are no obvious synapomorphies shared by these groups other than molecular data.
Illicium is sister to the [Kadsura+Schisandra] clade. A large number of morphological-anatomical (vessel perforation plates, nodes, sclereid morphology, foliar ptyxis, leaf teeth, androecial and pollen morphology, embryology, fruit type, seed morphology, etc.) and phytochemical synapomorphies connect Kadsura with Schisandra, and certain analyses of trnL-F and ITS sequences even indicate that Kadsura may be paraphyletic relative to Schisandra.
End walls of vessel elements in Illicium often possess remainders of pit membrane (Carlquist & Schneider 2002). The variation is extensive and transition forms between tracheids and vessels are frequent.
The carpel margins are not fused in Schisandra and Kadsura and the decurrent stigmatic areas have been classified as Wet type. The pollen tubes grow extracellularly on stigmatic epidermis cells and along cells of the abaxial epidermis, subsequently along the extragynoecial mucilaginous compitum and between the free carpels. They then penetrate a nearby carpel. In Illicium, on the other hand, the stigma is of the Wet type and the carpel is at least partially closed. The pollen tubes in Illicium grow through a short substigmatic zone, consisting of fused ground tissue, before reaching the stylar canal. The Schisandraceae, as well as several other basal angiosperm clades, have a carpellary transmitting tissue that resembles the one in stigmas of the Dry type (Lyew & al. 2007).
TRIMENIACEAE (J. R. Perkins et Gilg) L. S. Gibbs |
Trimeniales Doweld in Byull. Mosk. Obshch. Ispyt. Prir., Biol. 105(5): 60. 9 Oct 2000; Trimenianae Doweld, Tent. Syst. Plant. Vasc.: xxiii. 23 Dec 2001
Genera/species 1/8–13
Distribution Southeastern Queensland, Sulawesi and the Moluccas east to Fiji, Samoa and the Marquesas Islands.
Fossils Fossil pollen grains, Cretacaeisporites scabratus, assigned to Trimeniaceae are known from the Albian to Cenomanian (mid-Cretaceous) of Brazil and the Turonian (Late Cretaceous) of western Africa.
Habit Bisexual, monoecious, andromonoecious, polygamomonoecious, dioecious, or polygamodioecious, evergreen trees, shrubs or lianas.
Vegetative anatomy Phellogen ab initio superficial. Primary stem with separate vascular bundles. Vessel elements with scalariform perforation plates; lateral pits scalariform, simple pits. Imperforate tracheary xylem elements fibre tracheids with simple pits, septate or non-septate. Wood rays uniseriate or multiseriate, heterocellular. Axial parenchyma absent or very scarce. Sieve tube plastids S type, with ten to 15 starch grains. Nodes 1:2, unilacunar with two leaf traces. Secondary phloem sometimes with sclereids. Crystals absent?
Trichomes Hairs unicellular or tricellular, uniseriate, eglandular.
Leaves Opposite, simple, entire, with ? ptyxis. Stipules and leaf sheath absent. Petiole vascular bundle transection arcuate, with two or four bundles. Venation pinnate, brochidodromous. Stomata paracytic. Cuticular wax crystalloids as irregular platelets (chemically dominated by nonacosan-10-ol?). Lamina often with transparent gland-dots. Ethereal oil cell complexes present on abaxial and adaxial surfaces. Mesophyll with mucilage cells and secretory idioblasts with ethereal oils. Leaf margin serrate, with chloranthoid or monimioid teeth, or entire.
Inflorescence Usually axillary (sometimes terminal), botryoid. Floral prophylls (bracteoles) numerous, grading into tepals.
Flowers Actinomorphic. Hypogyny. Tepals two to 38, usually spiral (sometimes decussate), with imbricate aestivation, sepaloid to bracteolar, early caducous, free; outer tepals pairwise. Nectaries absent. Disc absent.
Androecium Stamens seven to c. 25, spiral. Filaments free from each other and from tepals. Anthers basifixed, non-versatile, tetrasporangiate, latrorse to extrorse (introrse?), longicidal (dehiscing by longitudinal slits); connective somewhat prolonged. Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis successive. Pollen grains inaperturate, disulculate or polyporate (with eight to twelve pores), shed as monads or tetrads, bicellular at dispersal. Exine tectate to semitectate, with usually columellate (sometimes granular) infratectum, perforate, rugulate or verrucate.
Gynoecium Pistil composed of usually a single carpel (rarely two carpels, more or less free); carpel ascidiate, occluded by secretion (carpel margins not fused); carpel with uniseriate hairs with tanniniferous elongate terminal cell; mucilaginous extragynoecial compitum present. Closure by transverse slit occurring together with longitudinal slit. Ovary superior, unilocular (pseudomonomerous [through abortion?]). Style absent. Stigma penicillate-hairy, Dry type, covered by mucilaginous layer. Male flowers often with pistillodium.
Ovules Placentation apical. Ovule one per carpel, anatropous, pendulous, bitegmic, crassinucellar. Micropyle bistomal. Outer integument five to seven cell layers thick. Inner integument two or three cell layers thick. Archespore multicellular. Megasporangium elongating following meiosis. Megagametophyte monosporous, quadrinucleate, Nuphar/Schisandra type. Pollen tubes growing directly into dense substigmatic ground tissue, subsequently reaching stylar canal due to breakdown of mid-lamellae. Endosperm development ab initio cellular. Endosperm haustoria? Embryogenesis?
Fruit A berry.
Seeds Seeds with an annular hood-shaped structure (‘cap’). Aril absent. Seed coat exotestal-mesotestal. Testa vascularized; almost all testal cell walls thick, lignified. Exotesta hard, with lignified cell walls. Mesotesta palisade; mesotestal cells sclerotic. Endotesta unspecialized. Tegmen unspecialized. Perisperm well developed. Endosperm copious, starchy and oily. Embryo small, straight, chlorophyll? Cotyledons two, rudimentary. Germination?
Cytology n = 8
DNA
Phytochemistry 5-O-methylflavonols, flavones, piptoside (a glycoside), lignans (veraguensin, calopiptin), and tiglic acid present. Benzylisoquinoline alkaloids not found. Aluminium accumulation occurring at least in some species.
Use Unknown.
Systematics Trimenia (8–13; southeastern Queensland, central Sulawesi, the Moluccas, New Guinea, New Britain, New Ireland, Bougainville, New Caledonia, Fiji, Samoa, the Marquesas Islands).
Trimenia is sister to Schisandraceae, although there are no clear synapomorphies other than molecular data. The microsporogenesis has been described as successive in Trimenia, but simultaneous in Austrobaileya and Schisandraceae.
Literature
Bailey IW, Nast CG. 1948. Morphology and relationships of Illicium, Schisandra, and Kadsura I. Stem and leaf. – J. Arnold Arbor. 29: 77-89.
Bailey IW, Swamy BGL. 1949. The morphology and relationships of Austrobaileya. – J. Arnold Arbor. 30: 211-226.
Baranova M. 1992. The epidermal structure and systematic position of Austrobaileyaceae. – Bot. Žurn. 77: 1-17. [in Russian]
Baranova M. 2004. The epidermal structure of Austrobaileya (Austrobaileyaceae) – a further comment. – Kew Bull. 59: 489-491.
Battaglia E. 1986. Embryological questions 7. Do new types of embryo sac occur in Schisandra? – Ann. Bot. 44: 69-82.
Behnke H-D. 1986. Sieve element characters and the systematic position of Austrobaileya (Austrobaileyaceae), with comments on the distinction and definition of sieve cells and sieve tube members. – Plant Syst. Evol. 152: 101-121.
Behnke H-D. 1988. Sieve-element plastids, phloem protein, and evolution of flowering plants III. Magnoliidae. – Taxon 37: 699-732.
Bernhardt P, Sage T, Weston P, Azuma H, Lam M, Thien LB, Bruhl J. 2003. The pollination of Trimenia moorei (Trimeniaceae): floral volatiles, insect/wind pollen vectors and stigmatic self-incompatibility in a basal angiosperm. – Ann. Bot. 92: 445-458.
Carlquist SJ. 1982. Wood anatomy of Illicium (Illiciaceae): phylogenetic, ecological, and functional interpretations. – Amer. J. Bot. 69: 1587-1598.
Carlquist SJ. 1984. Wood anatomy of Trimeniaceae. – Plant Syst. Evol. 144: 103-118.
Carlquist SJ. 1999. Wood and bark anatomy of Schisandraceae: implications for phylogeny, habit, and vessel evolution. – Aliso 18: 45-55.
Carlquist SJ. 2001. Observations on the vegetative anatomy of Austrobaileya: habital, organographic, and phylogenetic conclusions. – Bot. J. Linn. Soc. 135: 1-11.
Carlquist SJ, Schneider EL. 2002. Vessels of Illicium (Illiciaceae): range of pit membrane presence in perforations and other details. – Intern. J. Plant Sci. 163: 755-768.
Denk T, Oh I-C. 2005 [2006]. Phylogeny of Schisandraceae based on morphological data: evidence from modern plants and the fossil record. – Plant Syst. Evol. 256: 113-145.
Dickison WC, Endress PK. 1983. Ontogeny of the stem-node-leaf vascular continuum of Austrobaileya. – Amer. J. Bot. 70: 906-911.
Dong X-Y, Liu Z, Saunders R, Chen Z-D. 2012. Floral ontogeny of Schisandra chinensis (Schisandraceae): implications for androecial evolution within Schisandra and Kadsura. – Plant Syst. Evol. 298: 713-722.
Endress PK. 1980. The reproductive structures and systematic position of the Austrobaileyaceae. – Bot. Jahrb. Syst. 101: 393-433.
Endress PK. 1983a. The early floral development of Austrobaileya. – Bot. Jahrb. Syst. 103: 481-493.
Endress PK. 1983b. Dispersal and distribution in some small archaic relic angiosperm families (Austrobaileyaceae, Eupomatiaceae, Himantandraceae, Idiospermoideae-Calycanthaceae). – Sonderb. Naturwiss. Ver. Hamburg 7: 201-217.
Endress PK. 1993. Austrobaileyaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 138-140.
Endress PK, Honegger R. 1980. The pollen of the Austrobaileyaceae and its phylogenetic significance. – Grana 19: 177-182.
Endress PK, Sampson FB. 1983. Floral structure and relationships of the Trimeniaceae (Laurales). – J. Arnold Arbor. 64: 447-473.
Field TS, Franks PJ, Sage TL. 2003. Ecophysiological shade adaptation in the basal angiosperm Austrobaileya scandens (Austrobaileyaceae). – Intern. J. Plant Sci. 164: 313-324.
Floyd SK, Friedman WE. 2001. Developmental evolution of endosperm in basal angiosperms: evidence from Amborella (Amborellaceae), Nuphar (Nymphaeaceae), and Illicium (Illiciaceae). – Plant Syst. Evol. 228: 153-169.
Friedman WE, Bachelier JB. 2013. Seed development in Trimenia (Trimeniaceae) and its bearing on the evolution of embryo-nourishing strategies in early flowering plant lineages. – Amer. J. Bot. 100: 906-915.
Friedman WE, Gallup WN, Williams JH. 2003. Female gametophyte development in Kadsura: implications for Schisandraceae, Austrobaileyales, and the early evolution of flowering plants. – Intern. J. Plant Sci. 164(Suppl.): S293-S305.
Frumin S, Friis EM. 1999. Magnoliid reproductive organs from the Cenomanian-Turonian of north-western Kazakhstan: Magnoliaceae and Illiciaceae. – Plant Syst. Evol. 216: 265-288.
Gabarayeva NI, Grigoryeva VV. 2003. Comparative study of the pollen wall development in Illicium floridanum (Illiciaceae) and Schisandra chinensis (Schisandraceae). – Taiwania 48. 147-167.
Gilg E, Schlechter R. 1919. Über zwei pflanzengeographisch interessante Monimiaceen aus Deutsch-Neu Guinea. – Engl. Bot. Jahrb. Syst. 55: 195-201.
Goldblatt P. 1974. A contribution to the knowledge of cytology in Magnoliales. – J. Arnold Arbor. 55: 453-457.
Goldblatt P, Briggs BG. 1979. Chromosome number in two primitive dicots Xymalos monospora (Monimiaceae) and Piptocalyx moorei (Trimeniaceae). – Ann. Missouri Bot. Gard. 66: 88-89.
Gregor H-J. 1981. Schisandra geissertii nova spec. – ein exotisches Element in Elsässer Pliozän (Sessenheim, Brunssumien). – Mitt. Badischen Landesver. Naturk. Natursch. 12: 241-247.
Guerrero A, Judd WS, Morris AB. 2004. A new species of Illicium subsection Parviflora (Illiciaceae) from the Massif de la Hotte, Haiti. – Brittonia 56: 346-352.
Hansen DR, Dastidar SG, Cai Z, Penaflor C, Kuehl JV, Boore JL, Ransen RK. 2007. Phylogenetic and evolutionary implications of complete chloroplast genome sequences of four early-diverging angiosperms: Buxus (Buxaceae), Chloranthus (Chloranthaceae), Dioscorea (Dioscoreaceae), and Illicium (Schisandraceae). – Mol. Phylogen. Evol. 45: 547-563.
Hao G, Saunders RMK, Chye M-L. 2000. A phylogenetic analysis of the Illiciaceae based on sequences of internal transcribed spacers (ITS) of nuclear ribosomal DNA. – Plant Syst. Evol. 223: 81-90.
Hao G, Chye M-L, Saunders RMK. 2001. A phylogenetic analysis of the Schisandraceae based on morphology and nuclear ribosomal (ITS) sequences. – Bot. J. Linn. Soc. 135: 401-411.
Hayashi Y. 1960. On the microsporogenesis and pollen morphology in the family Magnoliaceae. – Sci. Rep. Tohoku Imp. Univ., 4th ser., Biology 26: 45-52.
Hayashi Y. 1963. The embryology of the family Magnoliaceae sensu lato I. Megasporogenesis, female gametophyte, and embryogeny of Schisandra repanda Radlkofer and Kadsura japonica Dunal. – Sci. Rep. Tohoku Imp. Univ. 4th ser., Biology 29: 27-33, 403-411.
Hegnauer R. 1986. Phytochemistry. – In: Philipson WR, Trimeniaceae, Flora Malesiana I, 10, Noordhoff, Leiden, p. 330.
Huynh K-L. 1976. L’arrangement du pollen du genre Schisandra (Schisandraceae) et sa significance phylogénique chez les angiospermes. – Beitr. Biol. Pflanzen 52: 227-253.
Jalan S. 1962. The ontogeny of the stomata in Schisandra grandiflora Hook. f. et Thoms. – Phytomorphology 12: 239-242.
Jalan S. 1965. Morphology and ontogeny of the ethereal oil cells in Schisandra Michaux. – Curr. Sci. 34: 527-528.
Jalan S. 1968a. Contribution to the nodal structure of Schisandra Michaux. – Bot. Jahrb. Syst. 88: 311-316.
Jalan S. 1968b. Observations on the crystalliferous sclereids of some Schisandraceae. – Beitr. Biol. Pflanzen 44: 277-288.
Jalan S, Kapil RN. 1964. Pollen grains of Schisandra Michaux. – Grana Palynol. 5: 216-221.
Kapil RN, Jalan S. 1964. Schisandra Michaux: its embryology and systematic position. – Bot. Not. 117: 285-306.
Keng H. 1965. Observations on the flowers of Illicium. – Bot. Bull. Acad. Sin., ser. II, 6: 61-73.
Keng H. 1993a. Illiciaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 344-347.
Keng H. 1993b. Schisandraceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 589-592.
Koehl V, Thien LB, Heij EG, Sage TL. 2004. The causes of self-sterility in natural populations of the relictual angiosperm Illicium floridanum (Illiciaceae). – Ann. Bot. 94: 43-50.
Lan S-F. 1984. Pollen morphology of the genus Kadsura in China. – J. South China Agricult. Coll. 5: 83-92. [In Chinese]
Lin Q. 1989. A study of the pollen morphology of genus Illicium L. – Bull. Bot. Res., Harbin 9: 115-124. [In Chinese]
Lin Q. 1997. Systematics and evolution of the family Illiciaceae. – Ph.D. diss., Forestry College, South China Agricultural University, Guangzhou, China. [In Chinese]
Lin Q. 2000. Taxonomic notes on the genus Schisandra Michx. – Acta Phytotaxon. Sin. 38: 532-550.
Liu H, Yang C-S. 1989. Pollen morphology of Illiciaceae and its significance in systematics. – Chin. J. Bot. 1: 104-115.
Liu Z, Hao G, Luo Y-B, Thien LB, Rosso SW, Lu A-M, Chen Z-D. 2006. Phylogeny and androecial evolution in Schisandraceae, inferred from sequences of nuclear ribosomal DNA ITS and chloroplast DNA trnL-F regions. – Intern. J. Plant Sci. 167: 539-550.
Lyew J, Li Z, Yuan L-C, Luo Y-B, Sage TL. 2007. Pollen tube growth in association with a dry-type stigmatic transmitting tissue and extragynoecial compitum in the basal angiosperm Kadsura longipedunculata. – Amer. J. Bot. 94: 1170-1182.
McAlpine JB, Riggs NV, Gordon PG. 1968. Absolute stereochemistry of calopiptin. – Aust. J. Chem. 21: 2095-2106.
Money LL, Bailey IW, Swamy BGL. 1950. The morphology and relationships of the Monimiaceae. – J. Arnold Arbor. 31: 372-404.
Morat P, MacKee HS. 1977. Quelques precisions sur le Trimenia neocaledonica Bak. f. et la famille des Triméniacées en Nouvelle-Calédonie. – Adansonia, sér. II, 17: 205-213.
Morawetz W. 1988. Karyosystematics and evolution of Australian Annonaceae as compared with Eupomatiaceae, Himantandraceae, and Austrobaileyaceae. – Plant Syst. Evol. 159: 49-79.
Morris AB, Bell CD, Clayton JW, Judd WS, Soltis DE, Soltis PS. 2007. Phylogeny and divergence time estimation in Illicium with implications for New World biogeography. – Syst. Bot. 32: 236-249.
Murphy ST, Ritchie E, Taylor WC. 1975. Some constituents of Austrobaileya scandens (Austrobaileyaceae): structures of seven new lignans. – Aust. J. Chem. 28: 81-90.
Oh I-C, Denk T, Friis EM. 2003. Evolution of Illicium (Illiciaceae): mapping morphological characters on the molecular tree. – Plant Syst. Evol. 240: 175-209.
Ozenda P. 1946. Sur l’anatomie libéroligneuse des Schizandracées. – Compt. Rend. Acad. Sci. Paris 223: 207-209.
Perkins J. 1925. Übersicht über die Gattungen der Monimiaceae. – W. Engelmann, Leipzig.
Philipson WR. 1986. Trimeniaceae. – In: Steenis CGGJ van, Wilde WJJO de (eds), Flora Malesiana I, 10(2), Martinus Nijhoff, The Hague, Boston, London, pp. 327-333.
Philipson WR. 1993. Trimeniaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 596-599.
Praglowski J. 1976. Schisandraceae Bl. – In: Nilsson S (ed), World Pollen and Spore Flora 5, Almqvist & Wiksell, Stockholm.
Prakash N, Alexander JH III. 1984. Self-incompatibility in Austrobaileya scandens. – In: Williams EG, Knox RB (eds), Pollination ’84, School of Botany, University of Melbourne, pp. 214-216.
Prantl K. 1891. Magnoliaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(2), W. Engelmann, Leipzig, pp. 12-19.
Roberts ML, Haynes RR. 1983. Ballistic seed dispersal in Illicium (Illiciaceae). – Plant Syst. Evol. 143: 227-232.
Robertson RE, Tucker SC. 1979. Floral ontogeny of Illicium floridanum, with emphasis on stamen and carpel development. – Amer. J. Bot. 66: 605-617.
Rodenburg WF. 1971. A revision of the genus Trimenia (Trimeniaceae). – Blumea 19: 3-15.
Romanov MS, Bobrov AVFC, Endress PK. 2013. Structure of the unusual explosive fruits of the early diverging angiosperm Illicium (Schisandraceae s.l., Austrobaileyales). – Bot. J. Linn. Soc. 171: 640-654.
Rüdenberg L. 1967. The chromosomes of Austrobaileya. – J. Arnold Arbor. 48: 241-244.
Sampson FB. 1987. Disulculate pollen in the Trimeniaceae. – Grana 26: 239-241.
Sampson FB, Endress PK. 1984. Pollen morphology in the Trimeniaceae. – Grana 23: 129-137.
Saunders RMKS. 1995. Systematics of the genus Illicium L. (Illiciaceae) in Malesia. – Bot. J. Linn. Soc. 117: 333-352.
Saunders RMK. 1997. Illiciaceae & Schisandraceae. – In: Kalkman C et al. (eds), Flora Malesiana, I, 13, Flora Malesiana Foundation, Rijksherbarium/Hortus Botanicus, Leiden, pp. 169-184, 185-207.
Saunders RMK. 1998. Monograph of Kadsura (Schisandraceae). – Syst. Bot. Monogr. 54: 1-106.
Saunders RMK. 2000. Monograph of Schisandra (Schisandraceae). – Syst. Bot. Monogr. 58: 1-148.
Saunders RMK. 2001. Schisandraceae. – Species Plantarum: Flora of the World 4, Australian Biological Resources Study.
Schneider EL, Carlquist SJ. 2003. Perforation plate diversity in Illicium floridanum with respect to organs, provenance, and microtechnical methods. – Sida 20: 1047-1057.
Smith AC. 1947. The families Illiciaceae and Schisandraceae. – Sargentia 7: 1-224.
Srivastava LM. 1970. The secondary phloem of Austrobaileya scandens. – Can. J. Bot. 48: 341-359.
Stone DE, Freeman JL. 1968. Cytotaxonomy of Illicium floridanum and I. parviflorum (Illiciaceae). – J. Arnold Arbor. 49: 41-51.
Swamy BGL. 1965. Macrogametophytic ontogeny in Schisandra chinensis. – J. Indian Bot. Soc. 43: 391-396.
Sy L-K, Saunders RMKS, Brown GD. 1997. Phytochemistry of Illicium dunnianum and the systematic position of the Illiciaceae. – Phytochemistry 44: 1099-1108.
Takahashi M. 1994. Exine development in Illicium religiosum Sieb. et Zucc. (Illiciaceae). – Grana 33: 309-312.
Thien LB, White DA, Yatsu LA. 1983. The reproductive biology of a relict – Illicium floridanum Ellis. – Amer. J. Bot. 70: 719-727.
Tiffney BH, Barghoorn ES. 1979. Flora of the Brandon Lignite IV. Illiciaceae. – Amer. J. Bot. 66: 321-329.
Tobe H, Kimoto Y, Prakash N. 2007. Development and structure of the female gametophyte of Austrobaileya scandens (Austrobaileyaceae). – J. Plant Res. 120: 431-436.
Tucker SC, Bourland JA. 1994. Ontogeny of staminate and carpellate flowers of Schisandra glabra (Schisandraceae). – Plant Syst. Evol. [Suppl.] 8: 137-158.
Ueda L. 1988. Sex change in a woody vine species, Schisandra chinensis, a preliminary note. – J. Jap. Bot. 63: 319-320.
Vijayaraghavan MK, Dhar U. 1975. Kadsura heteroclita – microsporangium and pollen. – J. Arnold Arbor. 56: 176-182.
Wagner WL, Lorence DH. 1999. A revision of Trimenia Seem. (Trimeniaceae) in the Marquesas Islands with description of a new species, Trimenia nukuhivensis. – Adansonia 21: 225-230.
Williams EG, Sage TL, Thien LB. 1993. Functional syncarpy by intercarpellary growth of pollen tubes in a primitive apocarpous angiosperm, Illicium floridanum (Illiciaceae). – Amer. J. Bot. 80: 137-142.
Williams JH, Friedman WE. 2002. Identification of diploid endosperm in an early angiosperm lineage. – Nature 415: 522-526.
Williams JH, Friedman WE. 2004. The four-celled female gametophyte of Illicium (Illiciaceae; Austrobaileyales): implications for understanding the origin and early evolution of monocotyledons, eumagnoliids, and eudicots. – Amer. J. Bot. 91: 332-351.
Yama T, Nishida H, Umebayashi M, Uemura K, Kato M. 2008. Oldest record of Trimeniaceae from the Early Cretaceous of northern Japan. – BMC Evol. Biol. 8: 135.
Yamada T, Kato M. 2008. Stopesia alveolata, a fossil seed of Trimeniaceae from the Lower Cretaceous (Albian) of Hokkaido, Northern Japan. – Acta Phytotaxon. Geobot. 59: 228-230.
Yamada T, Imaichi R, Prakash N, Kato M. 2003. Developmental morphology of ovules and seeds of Austrobaileyales. – Aust. J. Bot. 51: 555-564.
Yang Z-R, Lin Q. 2005. Comparative morphology of the leaf epidermis in Schisandra (Scisandraceae). – Bot. J. Linn. Soc. 148: 39-56.
Yang Z-R, Lin Q. 2007. Comparative wood anatomy of Schisandraceae and its systematic significance. – Acta Phytotaxon. Sin. 45: 191-206. [in Chinese]
Yoshida O. 1962. Embryologische Studien über Schisandra chinensis Baillon. – J. Coll. Arts Sci., Chiba Univ., 4: 459-462.
Yuan L-C, Luo Y-B, Thien LB, Fan J-H, Xu H-L, Chen Z-D. 2007. Pollination of Schisandra henryi (Schisandraceae) by female, pollen-eating Megommata species (Cecidomyiidae, Diptera) in South-Central China. – Ann. Bot. 99: 451-460.
Yuan L-C, Luo Y-B, Thien LB, Fan J-H, Xu H-L, Yukawa J, Chen Z-D. 2008. Pollination of Kadsura longipedunculata (Schisandraceae) a monoecious basal angiosperm, by female, pollen-eating Megommata sp. (Cecidomyiidae: Diptera) in China. – Biol. J. Linn. Soc. 93: 523-536.
Zavada MS. 1984. Pollen wall development of Austrobaileya maculata. – Bot. Gaz. (Crawfordsville) 145: 11-21.
Zhong L, Wang X-Q, Chen Z-D, Lin Q, Lu A-M. 2000. The phylogeny of Schisandraceae inferred from sequence analysis of the nrDNA ITS region. – Acta Bot. Sin. 42: 758-761. [in Chinese]