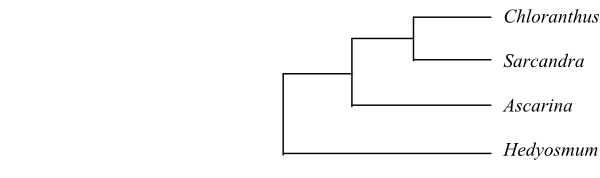
Cladogram of Chloranthaceae based on morphology and DNA sequence data (Zhang & Renner 2003, Soltis & al. 2011; Zhang & al. 2011).
[[Chloranthaceae+Magnoliidae]+[Liliidae+[Ceratophyllum+Tricolpatae]]]
[Chloranthaceae+[[Magnoliales+Laurales]+[Canellales+Piperales]]]
Chloranthales R. Br. in C. F. P. von Martius, Consp. Regn. Veg.: 12. Sep-Oct 1835 [‘Chlorantheae’]; Hedyosmaceae Caruel in Atti Reale Accad. Lincei, Mem. Cl. Sci. Fis., ser. 3, 10: 214, 249. 5 Jun 1881; Chloranthanae Doweld, Tent. Syst. Plant. Vasc.: xxiv. 23 Dec 2001; Chloranthidae C. Y. Wu in Acta Phytotaxon. Sin. 40: 292. 2002
Genera/species 4/>75
Distribution Madagascar, tropical Himalaya, southern, eastern and southeastern Asia, Borneo, New Guinea, Melanesia to the Marquesas Islands, North Island of New Zealand, tropical America.
Fossils Cretaceous and Cenozoic fossil Chloranthaceae are abundant on most continents. The pollen form genus Asteropollis comprises trichotomocolpate to hexachotomocolpate grains similar to those in extant Hedyosmum (and Ascarina) and known from the Barremian (possibly also Hauterivian; Early Cretaceous, c. 125 Mya) of Central Asia, and later from North and South America, Africa, Europe and Australia. The pollen fossil Clavatipollenites hughesii from Barremian to Aptian layers in England has frequently been compared to Ascarina. Fossil flowers strongly resembling Hedyosmum have been described from the Late Barremian to the Early Albian in Portugal and Virginia. Fossil stamens and fruits very similar to Chloranthus and Sarcandra have been found in mid-Cretaceous layers in Europe. Hammenia is a stephanocolpate Cretaceous pollen with reticulate exine, which resembles species of Chloranthus, Hedyosmum and Sarcandra. Chloranthistemon represents fossilized bisexual atepalous flowers from Late Santonian to Early Campanian of North America and Europe, which are strikingly similar to Chloranthus.
Habit Bisexual (Chloranthus, Sarcandra), monoecious or dioecious (Ascarina, Hedyosmum), evergreen trees or shrubs, or perennial herbs (sometimes woody at base, rarely annual). Aromatic.
Vegetative anatomy Roots in seedlings and young plants probably without secondary thickening. Phellogen? Primary stem with vascular cylinder. Endodermis present in stem. Vessel elements with scalariform perforation plates, or absent; lateral pits scalariform, opposite, transitional, or absent (in Sarcandra vessels are absent [secondarily?] except in roots), simple and/or bordered pits. Vestured pits present in Ascarina. Imperforate tracheary xylem elements tracheids, fibre tracheids or libriform fibres (in Hedyosmum) with bordered pits, septate or non-septate. Wood rays heterocellular, uniseriate or multiseriate. Axial parenchyma apotracheal diffuse or diffuse-in-aggregates, or paratracheal vasicentric scanty, or often absent. Wood soft (storied?). Sieve tube plastids S type, with five to 20 unequally sized starch grains. Nodes 1:1–6, unilacunar with one to six leaf traces, with or without divided lateral vascular bundles, or 3:3, trilacunar with three traces; two traces arising from one central or from all leaf gaps or divided lateral; often strongly swollen (often collapsing when dry). Cortex and pith with idioblasts containing ethereal oils. Mucilage canals present in, e.g., Hedyosmum. Calciumoxalate crystals absent.
Trichomes Hairs unicellular or multicellular, uniseriate or multiseriate, or absent.
Leaves Opposite, simple, entire, connate at petiole base, in Chloranthus with conduplicate ptyxis. Stipules small, interpetiolar, usually inserted on margin of sheathingly expanded petiole bases. Petiole vascular bundles? Venation pinnate, brochidodromous, eucamptodromous or semicraspedodromous. Stomata usually laterocytic, paracytic or cyclocytic (sometimes anomocytic, actinocytic or amphicyclic). Cuticular wax crystalloids as large and small platelets or waxes absent. Mesophyll with idioblasts containing ethereal oils. Mucilaginous canals present in, e.g., Hedyosmum. Sclereids present in Hedyosmum. Leaf margin serrate or crenate; marginal teeth chloranthoid, with persistent transparent swollen hood-like structure, into which lateral veins are running, sometimes with glandular tip and hydathode, sometimes with sclerotic apex.
Inflorescence Terminal or axillary, raceme, spike-, or head-like, or thyrsoid. Floral pherophylls (bracteoles) usully two (absent in male flowers of Hedyosmum).
Flowers Zygomorphic to asymmetric, very small. Epigyny or half epigyny. Tepals usually absent (in female flowers of Hedyosmum three, whorled, sepaloid, connate). Nectaries absent. Disc absent.
Androecium Stamen usually one, abaxial, or stamens three (in Ascarina up to five), two of which in different ways reduced, in Chloranthus and Sarcandra inserted beside gynoecium (in Chloranthus trilobate, surrounding gynoecium). Filaments very short, connate, free from tepals. Anthers basifixed, non-versatile, usually tetrasporangiate (in Chloranthus sometimes also disporangiate), introrse to latrorse, longicidal (dehiscing by longitudinal slits); in most species of Chloranthus either one to three, fused with a median bilocular anther and usually two lateral unilocular anthers, or two lateral unilocular anthers and usually no median anther; connective absent or present, sometimes prolonged or expanded. Tapetum secretory. Staminodia absent.
Pollen grains Microsporogenesis simultaneous. Pollen grains in Hedyosmum usually pentachotomo- or hexachotomosulcate (sometimes inaperturate), in Sarcandra polyforate (polyporate), in Ascarina monosulcate, in Chloranthus tetra- to hexacolpate, shed as monads, bicellular at dispersal. Exine tectate or semitectate, with columellate infratectum, perforate, reticulate or microreticulate, verrucate.
Gynoecium Pistil composed of a single carpel (monocarpellate); carpel ascidiate, postgenitally occluded by secretion (carpel margins not fused), slightly open at anthesis. Closure by transverse slit occurring together with longitudinal slit. Ovary inferior or semi-inferior, unilocular. Style single, simple, short, filled with secretions. Stigma subcapitate, clavate or linear (sometimes prolongate), non-papillate and Dry type, or papillate and Wet type. Pistillodium absent.
Ovules Placentation apical. Ovule one per ovary, usually orthotropous (in Chloranthus suborthotropous), pendulous, usually bitegmic, crassinucellar. Micropyle bistomal or endostomal. Outer integument usually four to eight (in Ascarina two) cell layers thick. Inner integument (three to) seven to ten cell layers thick. Nucellar cap present. Megagametophyte monosporous, Polygonum type. Antipodal cells persistent, sometimes proliferating. Endosperm development cellular. Endosperm haustoria? Embryogenesis onagrad (Sarcandra) or chenopodiad.
Fruit Usually a berry (in Hedyosmum a drupe) with accrescent and fleshy bracts.
Seeds Aril absent. Seed coat testal and usually tegmic. Testa in Chloranthus sometimes multiplicative. Inner exotestal epidermis with lignified cell walls. Mesotesta unspecialized, in Chloranthus lignified. Endotesta usually palisade, lignified, with calciumoxalate crystals and a fibrillar endoreticulum. Tegmen more or less crushed; exotegmen and mesotegmen fibrous; endotegmen ab initio subpalisade (tegmen in Hedyosmum unspecialized). Perisperm not developed. Endosperm copious, oily (in Sarcandra also starchy and proteinaceous, with aggregated starch grains). Embryo small, little or well differentiated, chlorophyll? (often as an undifferentiated mass of cells; embryo in Chloranthus without cotyledons at fruit shedding; organogenesis in Hedyosmum occurring during germination). Cotyledons two, small. Germination phanerocotylar (Chloranthus).
Cytology n = 8, 14, 15, 30
DNA
Phytochemistry Flavonol- and flavone-O- and -C-glycosyl derivatives, lignoids (simple coumarins), eudesmanolide sesquiterpene lactones, germacrane-like compounds, cinnamoyl amides and neolignans present. (S)norcolaurine synthase activity high suggesting presence of benzylisoquinoline alkaloids. Flavonols, ellagic acid and proanthocyanidins not found.
Use Medicinal plants, tea, fruits (Hedyosmum mexicanum), ornamental plants.
Systematics Hedyosmum (c 45; Central and South America, the West Indies, one species, H. orientale, in southern China and Southeast Asia), Ascarina (12; Madagascar, Borneo and East Malesia to Melanesia, New Zealand, the Marquesas and other southwestern Pacific islands), Chloranthus (18; southern and eastern Asia), Sarcandra (3; S. glabra, S. grandifolia, S. irvingbaileyi; India, China, Japan, Southeast Asia, Malesia to New Guinea).
Chloranthaceae are sister to Ceratophyllum in a four-gene analysis of mitochondrial DNA (Qiu & al. 2010) and in analyses of the plastid inverted repeat (Moore & al. 2011). The clade [Ceratophyllum+ Chloranthaceae] was sister to Tricolpatae. However, in many recent angiosperm phylogenies Chloranthaceae receives comparatively high support as sister to Magnoliidae [[Magnoliales+ Laurales]+[Canellales+Piperales]] (e.g. 85% bootstrap support in the 17 genes and 640 taxa analysis by Soltis & al. 2011). The indicated presence of benzylisoquinoline alkaloids would be a further support of this sister-group relationship.
Ascarina and Hedyosmum are usually monoecious or dioecious woody plants. Chloranthus and Sarcandra are herbs or suffrutices (semi-shrubs) with bisexual flowers. DNA sequences and morphology analyses resulted in the topology [Hedyosmum+[Ascarina+[Chloranthus+ Sarcandra]]] (Zhang & Renner 2003).
The end walls of vessel elements possess remnants of pit membranes in investigated species of Ascarina, Chloranthus, Hedyosmum and Sarcandra (Carlquist 1987, 1990, 1992a, 1992b). Transition forms frequently occur between true vessels and tracheids and vessel-like tracheary elements may have extensive primary wall material on their end walls.
The pollen tubes do not penetrate the substigmatic ground tissue, but grow inwards between densely situated epidermal papillae in the stigma and the stylar canal.
Hedyosmum, a clade with c. 45 species nearly all of them neotropical, is sister-group to the remaining Chloranthaceae. Presumed fossils of Hedyosmum and/or Ascarina have been found in Early Cretaceous layers of most continents. In a study using molecular clock, fossils, molecular phylogenetic analyses and ancestral area reconstruction, Antonelli & Sanmarín (2011) estimated the age of the Hedyosmum crown group to 36 to 43 million years and the Chloranthaceae crown group to 110 to 112 million years. On the other hand, the age of the Chloranthaceae stem group divergence has been estimated to roughly 125 to 170 million years (depending on methods used). Paleobotanical data suggest that Chloranthaceae were much more diverse during the Cretaceous, and the ancestor of Hedyosmum was widespread on the Northern Hemisphere during the Late Cretaceous. The recent relatively low diversity of Chloranthaceae may depend on large extinction rate due to climatic fluctuations. The rise of the northern Andes during the Miocene and Pliocene may have been responsible of the rapid diversification of Hedyosmum and its colonization of the Neotropics from the north.
 |
Cladogram of Chloranthaceae based on morphology and DNA sequence data (Zhang & Renner 2003, Soltis & al. 2011; Zhang & al. 2011). |
Literature
Aiso H, Ishiguri F, Takashima Y, Iizuka K,
Yokota S. 2014. Reaction wood anatomy in a vessel-less angiosperm Sarcandra
glabra. – IAWA J. 35: 116-126.
Antonelli A, Sanmartín I. 2011. Mass extinction, gradual cooling, or rapid radiation? Reconstructing the spatiotemporal evolution of the ancient angiosperm genus Hedyosmum (Chloranthaceae) using empirical and simulated approaches. – Syst. Biol. 60: 596-615.
Balthazar M von, Endress PK. 1999. Floral bract function, flowering process and breeding systems of Sarcandra and Chloranthus (Chloranthaceae). – Plant Syst. Evol. 218: 161-178.
Baranova M. 1983. On the laterocytic stomatotype in angiosperms. – Brittonia 35: 93-102.
Behnke H-D. 1988. Sieve-element plastids, phloem protein, and evolution of flowering plants III. Magnoliidae. – Taxon 37: 699-732.
Blanc P. 1986. Edification d’arbres par croissance d’établissement de type monocotylédonien: l’exemple de Chloranthaceae. – In: Colloque international sur l’arbre, Naturalia Monspeliensia, numéro hors série, Montpellier, pp. 101-123.
Carlquist SJ. 1987. Presence of vessels in wood of Sarcandra (Chloranthaceae): comments on vessel origins in angiosperms. – Amer. J. Bot. 74: 1765-1771.
Carlquist SJ. 1990. Wood anatomy of Ascarina (Chloranthaceae) and the tracheid-vessel element transition. – Aliso 12: 667-684.
Carlquist SJ. 1992a. Wood anatomy of Hedyosmum (Chloranthaceae) and the tracheid-vessel element transition. – Aliso 13: 447-462.
Carlquist SJ. 1992b. Wood anatomy and stem of Chloranthus; summary of wood anatomy of Chloranthaceae, with comments on relationships, vessellessness, and the origin of monocotyledons. – IAWA Bull., N. S., 13: 3-16.
Chlonova AF, Surova TD. 1988. Pollen wall ultrastructure of Clavatipollenites incisus Chlonova and two modern species of Ascarina (Chloranthaceae). – Pollen Spores 30: 29-44.
Crane PR, Friis EM, Pedersen KR. 1989. Reproductive structure and function in Cretaceous Chloranthaceae. – Plant Syst. Evol. 165: 211-226.
D’Arcy WG, Liesner RL. 1981. Hedyosmum (Chloranthaceae) in Panama. – Syst. Bot. 6: 74-86.
Doria MG, Pabón-Mora N, Gonzaález F. 2012. Reassessing inflorescence and floral morphology and development in Hedyosmum (Chloranthaceae). – Intern. J. Plant Sci. 173: 735-750.
Doyle JA, Eklund H, Herendeen PS. 2003. Floral evolution in Chloranthaceae: implications of a morphological phylogenetic analysis. – Intern. J. Plant Sci. 164(Suppl.): S365-S382.
Edwards JG. 1920. Flower and seed of Hedyosmum nutans. – Bot. Gaz. 70: 409-424.
Eklund H. 1999. Big survivors with small flowers. Fossil history and evolution of Laurales and Chloranthaceae. – Ph.D. diss., Acta Univ. Upsal., Uppsala.
Eklund H, Friis EM, Pedersen KR. 1997. Chloranthaceous floral structures from the Late Cretaceous of Sweden. – Plant Syst. Evol. 207: 13-42.
Eklund H, Doyle JA, Herendeen PS. 2004. Morphological phylogenetic analysis of living and fossil Chloranthaceae. – Intern. J. Plant Sci. 165: 107-151.
Endress PK. 1971. Bau der weiblichen Blüten von Hedyosmum mexicanum Cordemoy (Chloranthaceae). – Bot. Jahrb. Syst. 91: 39-60.
Endress PK. 1987. The Chloranthaceae: reproductive structures and phylogenetic position. – Bot. Jahrb. Syst. 109: 153-226.
Engler A. 1889. Chloranthaceae. – In: Engler A, Prantl K (eds), Die natürlichen Pflanzenfamilien III(1), W. Engelmann, Leipzig, pp. 12-14.
Friis EM, Crane PR, Pedersen KR. 1986. Floral evidence for Cretaceous chloranthoid angiosperms. – Nature 320: 163-164.
Hansen DR, Dastidar SG, Cai Z, Penaflor C, Kuehl JV, Boore JL, Ransen RK. 2007. Phylogenetic and evolutionary implications of complete chloroplast genome sequences of four early-diverging angiosperms: Buxus (Buxaceae), Chloranthus (Chloranthaceae), Dioscorea (Dioscoreaceae), and Illicium (Schisandraceae). – Mol. Phylogen. Evol. 45: 547-563.
Herendeen PS, Crepet WL, Nixon KC. 1993. Chloranthus-like stamens from the Upper Cretaceous of New Jersey. – Amer. J. Bot. 80: 865-871.
Hristova K, Lam M, Field T, Sage TL. 2005. Transmitting tissue ECM distribution and composition, and pollen germinability in Sarcandra glabra and Chloranthus japonicus (Chloranthaceae). – Ann. Bot. 96: 779-791.
Jérémie J. 1980. Notes sur le genre Ascarina (Chloranthaceae) en Nouvelle-Calédonie et à Madagascar. – Adansonia, sér. II, 20: 273-285.
Khlonova AF, Surova TD. 1988. Comparative analysis of sporoderm ultrastructure of Clavatipollenites incisus and two species of Ascarina (Chloranthaceae). – Bot. Žurn. 73: 305-314. [In Russian]
Kong H-Z. 2000. Karyotypes of Sarcandra Gardn. and Chloranthus Swartz (Chloranthaceae) from China. – Bot. J. Linn. Soc. 133: 327-342.
Kong H-Z. 2001. Comparative morphology of leaf epidermis in the Chloranthaceae. – Bot. J. Linn. Soc. 136: 279-294.
Kong H-Z, Lu AM, Endress PK. 2002. Floral organogenesis of Chloranthus sessilifolius, with special emphasis on the morphological nature of the androecium of Chloranthus (Chloranthaceae). – Plant Syst. Evol. 232: 181-188.
Kong H-Z, Chen Z-D, Lu A-M. 2002. Phylogeny of Chloranthus (Chloranthaceae) based on nuclear ribosomal ITS and plastid trnL-F sequence data. – Amer. J. Bot. 89: 940-946.
Kuprianova LA. 1967. Palynological data for the history of the Chloranthaceae. – Pollen Spores 9: 95-100.
Leroy JF. 1983a. Interprétation nouvelle des appareils sexuels chez les Chloranthacées (Chloranthales, Magnoliidées). – Compt. Rend. Acad. Sci. Paris, sér. 53, 296: 747-752.
Leroy JF. 1983b. The origin of angiosperms: an unrecognized ancestral dicotyledon, Hedyosmum (Chloranthales), with a strobiloid flower is living today. – Taxon 32: 169-175.
Li G-S, Meng Z, Kong H-Z, Chen Z-D, Theissen G, Lu A-M. 2005. Characterisation of candidate class A, B and E, floral homeotic genes from the perianthless basal angiosperm Chloranthus spicatus (Chloranthaceae). – Devel. Genet. Evol. 215: 437-449.
Ludlow-Wiechers B, Martínez-Hernández E. 1978. Catálogo palinológico para la flora de Veracruz 1. Palinología de la familia Chloranthaceae de Veracruz. – Biotica 3: 3-8.
Maekawa F. 1970. Notes on the stamens of Chloranthus japonicus. – J. Jap. Bot. 45: 289-294.
Maekawa F. 1971. Further notes on the stamens of Chloranthus japonicus. – J. Jap. Bot. 46: 198.
Martínez C, Madri~´an S, Zavada M, Jaramillo CA. 2013. Tracing the fossil pollen record of Hedyosmum (Chloranthaceae), an old lineage with recent Neotropical diversification. – Grana 52: 161-180.
Moore LB. 1977. The flowers of Ascarina lucida Hook. f. (Chloranthaceae). – New Zealand J. Bot. 15: 491-494.
Nakazawa K. 1956. Vascular course of Piperales I. Chloranthaceae. – Jap. J. Bot. 15: 199-207.
Occhioni P. 1954. Contribução ao estudo da familia Chloranthaceae com especial referência ao gênero Hedyosmum Sw. – Rio de Janeiro.
Patel RN. 1975. Wood anatomy of the dicotyledons indigenous to New Zealand 10. Chloranthaceae. – New Zealand J. Bot. 13: 141-148.
Qiu Y-L, Li L, Wang B, Xue J-Y, Hendry TA, Li R-Q, Brown JW. 2010. Angiosperm phylogeny inferred from sequences of four mitochondrial genes. – J. Syst. Evol. 48: 391-425.
Schulze H. 1900. Beiträge zur Anatomie des Blattes bei den Chloranthaceen. – Beih. Bot. Centralbl. 9: 81-85.
Skutch AF. 1927. Peculiarities in the structure of the stem, related to the leaf-sheath, in Hedyosmum. – Ann. Bot. 41: 715-730.
Smith AC. 1976. Studies of Pacific Island plants XXXIII. The genus Ascarina (Chloranthaceae) in the Southern Pacific. – J. Arnold Arbor. 57: 405-425.
Stuchlick L. 1984. Morfología de los granos de pollen de las Chlorantaceae y Canellaceae Cubanas. – Acta Bot. Hung. 30: 321-328.
Swamy BGL. 1950. Sarcandra, a vesselless genus of the Chloranthaceae. – J. Arnold Arbor. 31: 117-129.
Swamy BGL. 1953a. The morphology and relationships of the Chloranthaceae. – J. Arnold Arbor. 34: 375-411.
Swamy BGL. 1953b. Sarcandra irvingbaileyi: a new species of vesselless dicotyledon from South India. – Proc. Natl. Inst. Sci. India, Sect. B, 19: 301-306.
Swamy BGL. 1953c. A taxonomic revision of the genus Ascarina Forst. – Proc. Natl. Inst. Sci. India, Sect. B, 19: 371-388.
Swamy BGL, Bailey IW. 1950. Sarcandra, a vesselless genus of Chloranthaceae. – J. Arnold Arbor. 31: 117-129.
Takahashi H, Tamura M. 1990. Occurrence of vessel elements in the stem of Sarcandra glabra. – J. Jap. Bot. 65: 81-86.
Thierry R. 1913. Contribution à l’étude anatomique des Chloranthacées. – Trav. Lab. Mat. Méd., Paris 9: 1-158.
Todzia CA. 1988a. Flora Neotropica. Monograph 48. Chloranthaceae. – New York Botanical Garden, Bronx, New York.
Todzia CA. 1988b. Four new species of Hedyosmum (Chloranthaceae) from South America. – Syst. Bot. 13: 21-31.
Todzia CA. 1990. 57. Chloranthaceae. – In: Harling G, Andersson L (eds), Flora of Ecuador 40, Nord. J. Bot., Copenhagen, pp. 1-31.
Todzia CA. 1993. Chloranthaceae. – In: Kubitzki K, Rohwer JG, Bittrich V (eds), The families and genera of vascular plants II. Flowering plants. Dicotyledons. Magnoliid, hamamelid and caryophyllid families, Springer, Berlin, Heidelberg, New York, pp. 281-287.
Todzia CA, Keating RC. 1991. Leaf architecture of the Chloranthaceae. – Ann. Bot. Gard. 78: 476-496.
Upchurch GR, Dilcher DL. 1990. Cenomanian angiosperm leaf megafossils, Dakota Formation, Rose Creek Locality, Jefferson County, southeastern Nebraska. – US Geol. Surv. Bull. 1915: 1-55.
Vakhrameev VA. 1991. Jurassic and Cretaceous floras and climates of the Earth. – Cambridge University Press, Cambridge.
Verdcourt B. 1984. The correct name for the Southern Indian and Sri Lankan species of Sarcandra Gardner (Chloranthaceae). – Kew Bull. 39: 66.
Verdcourt B. 1985. Notes on Malesian Chloranthaceae. – Kew Bull. 40: 213-224.
Verdcourt B. 1986. Chloranthaceae. – In: Steenis CGGJ van, Wilde WJJO de (eds), Flora malesiana I, 10(2), Martinus Nijhoff, The Hague, Boston, London, pp. 123-144.
Vijayaraghavan MR. 1964. Morphology and embryology of a vesselless dicotyledon, Sarcandra irvingbaileyi Swamy, and its systematic position in the Chloranthaceae. – Phytomorphology 14: 429-441.
Yamada T, Tobe H, Imaichi R, Kato M. 2001. Developmental morphology of the ovules of Amborella trichopoda (Amborellaceae) and Chloranthus serratus (Chloranthaceae). – Bot. J. Linn. Soc. 137: 277-290.
Yoshida O. 1957. Embryologische Studien über die Ordnung Piperales I. Embryologie von Chloranthus japonicus. – J. Coll. Arts Chiba Univ. 2: 172-178.
Yoshida O. 1959. Embryologische Studien über die Ordnung Piperales II. Embryologie von Chloranthus serratus. – J. Coll. Arts Chiba Univ. 2: 295-303.
Yoshida O. 1960. Embryologische Studien über die Ordnung Piperales III. Embryologie von Sarcandra glabra. – J. Coll. Arts Chiba Univ. 3: 55-60.
Zhang L-B, Renner SS. 2003. The deepest splits in Chloranthaceae as resolved by chloroplast sequences. – Intern. J. Plant Sci. 164(Suppl.): S383-S392.
Zhang Q, Antonelli A, Feild TS, Kong H-Z. 2011. Revisiting taxonomy, morphological evolution, and fossil calibration strategies in Chloranthaceae. – J. Syst. Evol. 49: 315-329.